| 1. | ||
| 2. | ||
| 3. | ||
Thermodynamic Analysis of Main Reactions in the Silicon-Titanium System During Self-Propagating High-Temperature Synthesis
A. S. Strogova, А. A. Kovalevskii, O. M. Komar
Belarusian State University of Informatics and Radioelectronics, Minsk, Republic of Belarus
Email address
 (A. S. Strogova)
(A. S. Strogova) Citation
A. S. Strogova, А. A. Kovalevskii, O. M. Komar. Thermodynamic Analysis of Main Reactions in the Silicon-Titanium System During Self-Propagating High-Temperature Synthesis. AASCIT Journal of Materials. Vol. 1, No. 4, 2015, pp. 123-127.
Abstract
Chemical reactions and mechanisms of structure formation in the high-temperature synthesis (SHS) of titanium disilicide are considered. It has been established that the course of exothermic reaction of the silicon-titanium system is determined by the process initiation temperature, the initial nanoscale of powder particles and titanium-to-silicon mass ratio.
Keywords
Titanium Disilicide, High-Temperature Synthesis, Nanostructured Materials
1. Introduction
The phenomenon of self-propagating high-temperature synthesis (SHS) of refractory compounds was discovered by Academician Merzhanov A.G. and his scientific school in 1967 [1,2]. The method is notable for low power inputs, simplicity and reliability of the equipment, cleanliness of the synthesized product. Currently, over a thousand of binary and multicomponent compounds have been synthesized by this method [1,2].
The SHS process control is one of the main tasks of fundamental and applied materials science, which purpose is obtaining monophasic product with the required chemical composition. In this regard, the mode of thermal explosion is more preferable because it admits the possibility of the batch thermal activity control by changing the external thermophysical conditions. It should be noted that up to date this issue has been insufficiently studied. There are no specific recommendations about carrying out the synthesis mode in one binary or multicomponent system or another. Therefore, the study of ways to control the SHS processes is an important and urgent task. Understanding of thermodynamics of possible chemical reactions and structure formation mechanisms in SH-synthesis is necessary for these purposes.
2. The Essence of SHS and Discussion of the Results
Exothermic reaction of the synthesis is initiated locally in the system consisting of the mixture of nanoscale powders of chemical elements (batch). The heat released as a result of the reaction owing to the heat transmission, heats the adjacent colder layers of the substance, excites reaction in them and leads to the appearance of self-propagating process.
In such process a chemical reaction proceeds in the narrow area which spontaneously moves along the substance with definite linear velocity. The high temperature required for quick reaction progress, is created as a result of release of the chemical energy stored in the source system. The reaction progress is accompanied by a series of parallel reactions, sometimes tending to one main thermodynamically favorable reaction.
The purpose of this work - thermodynamic analysis of main reactions in the silicon-titanium system during self-propagating high-temperature synthesis (SHS).
The analysis of any chemical transformation is based on an idea of energy. It is not sufficient to write it down as a chemical equation if there is no information about occurring chemical energy changes. It is impossible to understand without this, whether this transformation can noticeably proceed in this chemical system and to what extent it will be balanced by the reverse reaction. The most general knowledge of thermodynamics is enough to understand the chemical processes. Of course, there are areas where it is not enough, but in general, the basic principles of thermodynamics, where thermodynamic equations are used, are enough [3–5].
As already stated, only energy change determines which chemical reactions are possible and which are not. We note that in a chemical system we are interested in the reactions proceeding with the formation of a large number of the product we need. In principle, thermodynamic factors do not interfere with full reaction passing (except in cases when the substrates and products are in equilibrium concentrations). But if the reaction stops after the minimum transformation of the substrate has occurred, then from the point of view of thermodynamics such reaction does not matter.
First of all we define what is meant by energy change of the chemical system, i.e. an ensemble of molecules involved in chemical reactions. It is not so easy to understand than, for example, the change of gravitational energy of falling bodies. The chemical system consists of a large number of individual molecules, in each of which a certain amount of energy is contained, which is determined by its structure. This energy can be represented as heat content or enthalpy of the molecule. When the molecule structure is changing during a chemical reaction, its energy change is described as the change of enthalpy (ΔH). It can be negative (heat is lost by the molecules and dissipated with increasing the environment temperature) or positive (heat is absorbed from the environment, which is cooled). At first glance it may seem that the reaction with positive ΔH is as unreal as the body spontaneously flying up. But such a simple physical analogy is not applicable to chemical reactions, because a sign of the enthalpy change does not define rigidly the direction of the process, but only shows that this factor either promotes the process or hinders it. The place where the body will fall, depends only on the change in gravitational energy, and what kind of reaction will follow depends not only on the change in enthalpy (ΔH), but also on the change in entropy (ΔS) of the chemical system. Entropy can be defined as the degree of disorder. Disorder manifests in the chemical system in different ways. As the molecule is usually not rigid structure, it can vibrate, rotate as a single unit and relative to its separate bonds. The easier any molecular motion occurs, the greater disorder or entropy is. Moreover, a large number of molecules forming a chemical system can be randomly dissipated in space or exist in it in one degree or another orderly, as it is the case in the SHS. Finally, the number of individual molecules and ions in the system can change as a result of chemical reactions. The greater the number of molecules is, the more their aggregate randomness is, and consequently the entropy of the system. Both ΔH and ΔS make their contribution while solving the problem, whether there will be a chemical reaction. The negative ΔH and positive ΔS together give an answer "yes", while the positive ΔH and negative ΔS – the answer "no". If the enthalpy and entropy changes have the same signs, their influence is opposite, and the problem is solved by comparing their values.
The general statement is that more disordered state of the system is preferable at comparable values of the energy.
When assessing the possibility of reaction to proceed it is extremely inconvenient to compare the change in enthalpy and entropy, which can be allies or antagonists, at least because their sizes have different dimensions. Furthermore, the direct measurement of entropy is difficult or impossible in SHS. However, the situation is much improving thanks to the free energy concept suggested by Gibbs, that combines both of the indicated concepts - enthalpy and entropy. Famous equation describes change of the free energy [3–5]:
∆G = ∆H - T∆S (1)
where T- absolute temperature.
The definition of free energy here does not mean freedom in general, but the freedom to use this energy for useful work. ΔG is the maximum energy value which is available for committing useful work at the expense of chemical reaction. In other words, it is the part of the energy which we can rely on in the chemical processes.
With respect to SHS, useful work is a structural transformation, chemical synthesis in the volume of nanostructured nanoscale powders, overcoming chemical or heat-and-power engineering forces. The free energy change in the course of any process is the most important thermodynamic parameter. With regard to the chemical processes a general rule can be formulated , a chemical reaction takes place only in the case of ΔG<0; i.e. under conditions where the free energy of reaction products is less than that of the starting materials.
As is known many chemical reactions are reversible [3]. It can puzzle, because if it turns out that the values of ΔG are negative for direct and reverse reaction (remember that this is the criterion of capability of spontaneous flowing of any process). The solution of the seeming paradox lies in the fact that the ΔG is not a fixed parameter and is dependent on the concentration of the starting substances and products of their conversion. We note that for a reversible reaction A↔B change of free energy ΔG can be negative conformably to the transformation A→B and positive conformably to the transformation B→A, if concentration of the substance A is greater than the concentration of the substance B. Change of free energy ΔG to the transformation of B→A may become negative during the reverse ratio of the concentrations of A and B. It is easy to see that there exists some ratio of concentrations at which the change ΔG for the forward and reverse reactions is equal to zero. Such ratio will remain as long as desired – this is the point of chemical equilibrium.
If the value of ΔG for some chemical reaction A↔B is not large modulo, then with high probability this reaction at SHS will be reversible, since change of concentration of the substances A and B in the SHS can lead to the reversal of the sign ΔG. Conversely, if the absolute value of the ΔG modulo is large, the reaction can be considered practically irreversible. The range of changes inside of the reaction concentrations of the constituent components (the term uniting all the participants of chemical reactions) is relatively narrow: the concentration values usually are in the range 10-4–10-3 mole (M). As a result the reactions with large modulo negative values of ΔG are really irreversible, because the concentration change is not enough for the reversal of the sign ΔG [3,4,6,7]. Chemical reactions are going in one direction during considerable change of free energy and really proceed till full completion, because the point of equilibrium is shifted to this particular area. In other words, the starting substances are fully converted to the reaction products.
Main important chemical processes typically include not one, but a lot of successive reactions in the formation of silicides during SH- synthesis. They are grouped into so-called successive ways where the base reaction product is the starting substance for the second reaction, etc. For example, there are at least three successive chemical reactions in the conversion of Ti and Si into Ti5Si3. The common property of the successive ways is their irreversibility. This does not mean that all the reactions included in them are irreversible. However, at least one reaction does not flow in the reverse direction in the conditions of SH-synthesis. These reactions, acting like a pipeline valve, provide a thermodynamic basis of completeness of the total chemical transformation. Furthermore, it does not imply chemical irreversibility of the resultant transformation. Thus, TiSi, TiSi2 and Ti5Si3 are formed as a result of SHS from titanium and silicon. As a result, both forward (titanium-silicon) and reverse (disilicide titanium - titanium silicide) successive ways are irreversible. A typical successive situation can be described by the following scheme: Ti + Si → TiSi*, TiSi + Si → TiSi2*, TiSi2 + TiSi + 3Ti → Ti5Si3*.
In this scheme, the irreversible reactions with large modulo negative values ∆GоТ are indicated by asterisks, where ∆GоТ are the calculated values of the Gibbs energy for a given temperature of synthesis (Figure 1).
Although the change of free energy in the chemical reaction is not constant in some specific conditions adopted for the standard, the value ∆Gо298 is constant. Concentrations of all substances-participants of SHS - 1.0 M, the temperature is 298 K and pH–7.0 (neutral ambient) are taken as such standard conditions. The value ∆Gо298 corresponding to these conditions is called the standard free energy change of this reaction and is denoted ∆Gо298.
Physicochemical tables [3, 4, 6 – 8], in which the standard free energies of formation of a large number of chemical compounds are represented, are often used to calculate the standard free energy change of various reactions. When you separately add up the values for the starting substances and reaction products, the difference between these two amounts will be the desired value ∆Gо298. Experimental determination of the equilibrium constant of this reaction, from which it is easy to calculate ∆GоТ, is an alternative. Simple relation ∆GоТ = −RT∙ln Кeq connects the values ∆GоТ and Кeq at given concentrations of the starting substances and reaction products [3]. Therefore, knowing the concentrations of the chemical reaction components during SHS we can calculate the free energy change which characterizes the corresponding chemical reaction by the equilibrium constant.
However, it is not easy to determine the concentration of a number of components within the scope of SHS components, the content of which is very low and not constant. Therefore, for many chemical reactions the value ∆Gо298 still remains unknown. Fortunately, it turned out that in many cases it is possible to use the known values ∆Gо298, although the conditions corresponding to them within the volume of the components at the SH-synthesis are not always implemented. Of course, this is a compromise, but the useful one. Knowing the values of ΔGoT is particularly useful because the equilibrium constant of the corresponding reaction can be easily calculated from them (Table 1).
Table 1. Equilibrium constants of the reactions of titanium silicides formation.
| Kp | |||
| T, К | Ti+Si→TiSi | TiSi+Si→TiSi2 | TiSi2+ TiSi + 3Ti →Ti5Si3 |
| 298 | 3,645∙1023 | 2524,03 | 1,879∙1051 |
| 300 | 2,561∙1023 | 2513,225 | 7,874∙1050 |
| 400 | 4,821∙1017 | 2197,347 | 5,503∙1036 |
| 600 | 6,148∙1011 | 2086,286 | 2,585∙1022 |
| 800 | 5,925∙108 | 2157,326 | 1,331∙1015 |
| 1000 | 8,204∙106 | 2280,317 | 4,758∙1010 |
| 1200 | 4,386∙105 | 2422,388 | 4,611∙107 |
| 1400 | 5,137∙104 | 2571,939 | 2,993∙105 |
| 1600 | 9,876∙103 | 2724,112 | 6,444∙103 |
| 1800 | 2655,202 | 2876,415 | 310,698 |
| 2000 | 905,496 | 3027,958 | 26,457 |
| 2200 | 367,948 | 3178,142 | 3,419 |
For providing chemical reaction it is necessary that the decrease of free energy corresponds to it. However, this does not mean that it will proceed with a noticeable rate. The assessment of the reaction of interacting silicon with titanium, i.e. silicidization with the formation of TiSi, TiSi2, Ti5Si3 by the reactions: Ti + Si → TiSi*, TiSi + Si→ TiSi2*, TiSi2 + TiSi +3Ti → Ti5Si3*, from the point of view of thermodynamics indicates that energetically it is very favorable process (Figure 1).
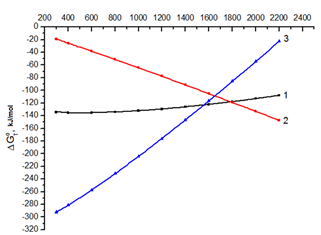
1– TiSi, 2 –TiSi2, 3 – Ti5Si3
Figure 1. Temperature dependence ΔGoT for the silicide formation reactions.
While calculating the free energy of the main reactions in the titanium-silicon system during SHS, the most promising ones from the point of view of making titanium silicides, the methodology was used according to which the free energy of the chemical reaction depends on the thermodynamic parameters of the initial reactants and final products, as well as on the process temperature [3]. The calculations are made on the basis of equations 1–7:
∆HT=∆H0298+∆Cp(T-298) (2)
∆ST=∆S0298+∆Cpln(T/298) (3)
∆GT=∆H0298+∆Cp(T-298) - T∆S0298 - T∆Cpln(T/298) (4)
∆H0298=∑(H0298 fin - H0298 basic) (5)
∆S0298=∑(S0298 fin - S0298 basic) (6)
∆Cp =∑(Cp fin - Cp basic) (7)
There ∆HоT, ∆S0T, ∆GоT - respectively, the change of enthalpy, entropy and Gibbs energy at a given temperature synthesis, ∆H0298, ∆S0298, ∆Cp– respectively, the change of enthalpy, entropy and molar heat capacity at room temperature.
The effectiveness of the chemical reactions in SHS is determined by the value ∆GоТ. The larger it is modulo, the more favorable reaction is in terms of efficiency of the process of a particular compound formation. The calculated values ΔGoT for possible silicides which are formed as a result of SH-synthesis, the most promising ones for use as photocatalysts, are shown in Figure 1. Analyzing the data obtained, it can be stated that in the conditions of SHS all the above-considered reactions are possible, especially in the area of low initiation temperatures 1070-1170 K, but in addition the advantage of this or that reaction is still determined by the ratio of interacting components (Figure 2). The probability of the reactions, the products of which are TiSi and Ti5Si3, is reduced, and the reaction to form TiSi2 with the mass fraction of titanium 0.86 and silicon 1.0 gains an advantage with the temperature increase. This is confirmed by the results of X-ray and mass spectrometric researches [9,10]. Thus, at the stage of calculating the thermodynamic parameters of reactions the final products of chemical interaction in the SH-synthesis conditions can be predicted with high degree of probability.
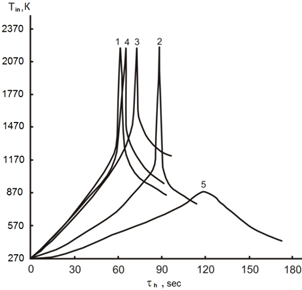
1 – 0,86 Ti + Si, Tin = 1270 К; 2 – 0,86 Ti + Si + 0.005 S, Тin = 1070 К;
3 – Ti + Si, Tin = 1420 К; 4 – 0,5Ti + Si, Тin = 1250 К; 5 – 086 Ti + Si, Тin = 870 К
Figure 2. The thermograms of self-heating of mixture in the coordinates of heating time (τh, s), initiation temperature (Тin, K) of those passed mechanoactivation τact = 12 min at volumetric ignition.
At the same time titanium and silicon are quite stable at room temperature. There exists a barrier for proceeding the reactions even with very large decrease of free energy, otherwise all the combustible materials on Earth would long ago have disappeared in the fire. However, titanium and silicon in such an inert environment rapidly react at high temperature and in the presence of oxygen or other oxidants, for example, sulfur [11]. The question arises: why the reactions proceeding in the SHS conditions, in other different conditions do not go at all, or go with insignificant small speeds. Because in the SHS conditions they are carried out as a result of exothermic reactions.
As is known, SHS accelerates advantageously only one chemical reaction. There are thousands of chemical reactions, and each of them is stimulated by its initiation temperature and the ratio of the reacting components [9,10]. The latter determines the direction of the formation of one or another by silicide composition. The principle of "one reaction - one initiating temperature" is observed in the case of multifunction chemical reactions with different chemical activity, such as, for example, nanostructured nanoscale materials, and in the case of multichemical complexes.
The nanostructured nanoscale powders of silicon and titanium, which were selected for research, were exposed to direct thermal heating. The research was performed using small amounts of sulfur for maximum practical effect.
Main nanoscale titanium silicides as the SHS synthesis products are semiconductors [9]. The important in this repect TiSi, TiSi2 and Ti5Si3 are built of the silicon and titanium atoms, connected in a certain order. These are essentially composite compounds, which consist of the smallest nanostructured silicides 10-50 nm, and many of them reach only a few nanometers, combined in agglomerates of up to 100 nm and conglomerates up to 150-300 nm (Figure 3).

а – ≥10 nm, b – <10 nm
Figure 3. Nanostructured silicides.
One of the reasons why silicides are so active is that the nanoscale fragments, of which they consist, convolve into a closed framework (Figure 3a) to form an active center, due to availability of great number of free dangling bonds.
Owing to this structure, the titanium disilicide is characterized by its high absorption capacity and high surface activity and is widely used as a photocatalyst in the synthesis of hydrogen during decomposition of water, as well as the hydrogen storage [9,10,12].
3. Conclusions
The effective way of creating the titanium disilicide with semiconducting properties - SH-synthesis- has been proposed and implemented based on the analysis of thermodynamic parameters. The effectiveness of the chemical reactions in SHS is determined by the value ∆GоТ. The larger it is modulo, the reaction is more favorable in terms of efficiency of the process of forming silicide.
The reality of nanostructured nanoscale silicon powders and titanium disilicide as a phase, which is stable under the conditions of formation of functional materials of widest profile, apparently, cannot give rise to doubts. A breakthrough in principally new fields of theoretical physics, photocatalysis, fuel and hydrogen energy, undoubtedly, will be connected with the research of nanostructured nanoscale powders.
List of Symbols
T – absolute temperature, К;
∆H – enthalpy change, kJ / mol;
∆S – entropy change, J /( mol К);
∆G – Gibbs free energy change, kJ / mol;
∆HоT – enthalpy change at predetermined temperature of synthesis, kJ / mol ;
∆S0T – entropy change at predetermined temperature of synthesis, J /( mol К);
∆GоT – Gibbs free energy change at predetermined temperature of synthesis, kJ / mol;
∆H0298 – enthalpy change at room temperature, kJ / mol;
∆S0298 – entropy change at room temperature, J /( mol К);
∆Cp – molar heat capacity change at room temperature, J / (mol К);
Keq – equilibrium constant;
R – universal gas constant, 8,314 J /( mol К);
τh – heating time of mixture, s;
Тin – initiation temperature, К;
τact – time of mechanical activation, min.
References