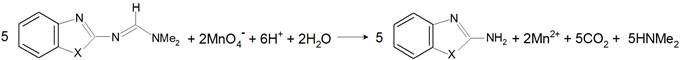Permanganate Oxidation of Benzimidazole and Benzthiazole Derivatives in Diluted Sulfuric Acid Medium: Kinetics and Mechanistic Aspects
Fawzy A.1, 2, *, Zaafarany I. A.1, Khairou K. S.1, Al-Jahdali B. A.1, Bawazeer T. M.1, Yarkandi N.1
1Chemistry Department, Faculty of Applied Sciences, Umm Al-Qura University, Makkah Al-Mukarramah, Saudi Arabia
2Chemistry Department, Faculty of Science, Assiut University, Assiut, Egypt
Email address

(Fawzy A.)
*Corresponding author
Citation
Fawzy A., Zaafarany I. A., Khairou K. S., Al-Jahdali B. A., Bawazeer T. M., Yarkandi N. Permanganate Oxidation of Benzimidazole and Benzthiazole Derivatives in Diluted Sulfuric Acid Medium: Kinetics and Mechanistic Aspects. Biochemistry and Molecular Biology. Vol. 1, No. 3, 2016, pp. 11-19.
Abstract
The kinetics of oxidation of N,N-dimethyl-N’-(1H-benzimidazol-2-yl) formamidine (BIF) and N,N-dimethyl-N’-(benzthiazol-2-yl) formamidine (BTF) by permanganate ion in diluted sulfuric acid medium has been investigated spectrophotometrically at a constant ionic strength of 0.2 mol dm-3 and at a temperature of 25°C. The reactions of both organic reductants with permanganate ion showed a first order dependence with respect to [MnO4−] and fractional-first order dependences with respect to both hydrogen ion and reductants concentrations. Increasing either ionic strength or dielectric constant of the reactions media had no significant effect on the oxidation rates. Manganese(II) ion was found to auto-catalyze the oxidation reactions with less than unit order dependences. The final oxidation products of BIF and BTF were identified by both spectroscopic and chemical tools as 2-aminobenzimidazole and 2-aminobenzthiazole, respectively, in addition to dimethylamine and carbon dioxide. Under comparable experimental conditions, the oxidation rate of BIF was higher than that of BTF. A plausible reactions mechanism has been suggested and the reaction constants involved in the mechanism have been evaluated. The activation parameters with respect to the second order rate constants have been computed and discussed.
Keywords
Benzimidazole and Benzthiazole Derivatives, Kinetics, Mechanism, Permanganate, Oxidation
1. Introduction
Benzimidazole and benzthiazole derivatives are significant heterocyclic organic compounds which have been a topic of interest for research for over a century because they have important biological activities and a broad spectrum of pharmaceutical and industrial applications. Benzimidazole structure is a part of the nucleotide portion of vitamin B12 and the nucleus in some drugs such as proton pump inhibitors and anthelmintic agents. Benzimidazole and its derivatives are widely employed as intermediates in the synthesis of vital organic compounds including pharmaceuticals, agrochemicals, dyes, photographic chemicals, corrosion inhibitors, epoxy curing agents, adhesives and plastic modifiers. Benzthiazole derivatives have been developed for the treatment of muscle relaxants, diabetes, tuberculosis, epilepsy, analgesia, inflammation and viral infection [1-3]. They were showed inhibitory effect against human laryngocarcinoma [4], anticancer [5,6], antitumoractivity [7], fungicidal activities [8], antihelmintic [9], antiviral [10], and antimicrobial activity [11]. The benzimidazolyl- and benzthiazolyl-formamidine derivatives reacts with heterocyclic amines to give biologically active heterocyclic compounds [12]. On the other hand, The oxidative cleavage of formamidine derivatives is quite important, since the N,N-dialkyl-formamidine group is one of the most versatile protecting groups, especially in biosyntheticapplications.
Oxidation reactions are considered as a significant field in organic synthesis. The permanganate ion is considered as the most important oxidizing agent in acidic, neutral and alkaline media. It is extensively used for oxidation of organic compounds [13-30], and it is an important source of mechanistic information. It is stable in neutral and slightly alkaline media, but it disproportionates in strongly alkaline media to form blue hypomanganate(V) and green manganate(VI), which are short-lived transient species [19-23].
Although the kinetics of oxidation of benzimidazolyl-formamidine (BIF) and benzthiazolyl-formamidine (BTF) derivatives by permanganate ions have been investigated in aqueous alkaline medium [16], there are no reports describing their oxidation kinetics by this oxidant in different media. A detailed study of the title reactions was therefore undertaken in order to understand the effect of the medium on the oxidation kinetics and mechanism.
2. Materials and Methods
2.1. Materials
All chemicals employed in the present work were of analytical grade and their solutions were prepared by dissolving the requisite amounts of the samples in doubly distilled water. Stock solutions of benzimidazolyl-formamidine (BIF) and benzthiazolyl-formamidine (BTF) derivatives were prepared as described elsewhere [16]. A fresh solution of potassium permanganate was prepared and standardized as reported [31]. Sulfuric acid and sodium sulfate were used to provide the required acidity and ionic strength, respectively, and acetic acid was used to study the effect of dielectric constant.
2.2. Kinetic Measurements
All kinetic measurements were performed under pseudo-first order conditions where a large excess of BIF or BTF was present over permanganate ion. The ionic strength of the reactions media was adjusted to 0.2 mol dm−3. The reactions temperature (25°C) was controlled to within ± 0.1°C. The solutions of permanganate oxidant and the mixtures containing BIF or BTF and sulfuric acid were separately thermostated for about 2 h. Then, the permanganate solution was added to the mixtures. The progress of the reactions was followed by recording the decrease in the absorbance of permanganate ion as a function of timeat its absorption maximum (λ = 525 nm), whereas the other constituents of the reactions mixtures did not absorb significantly at this wavelength as shown in Fig. 1. The absorbance measurements were made in a thermostatted Shimadzu UV-VIS-NIR-3600 double-beam spectrophotometer. Time-resolved spectra during the oxidation of BIF and BTF by permanganate ion in diluted sulfuric acid solutions were illustrated in Fig. 1a, b, respectively. The Figure shows gradual disappearance of the permanganate band at l = 525 nm as a result of its reduction by organic substrates.
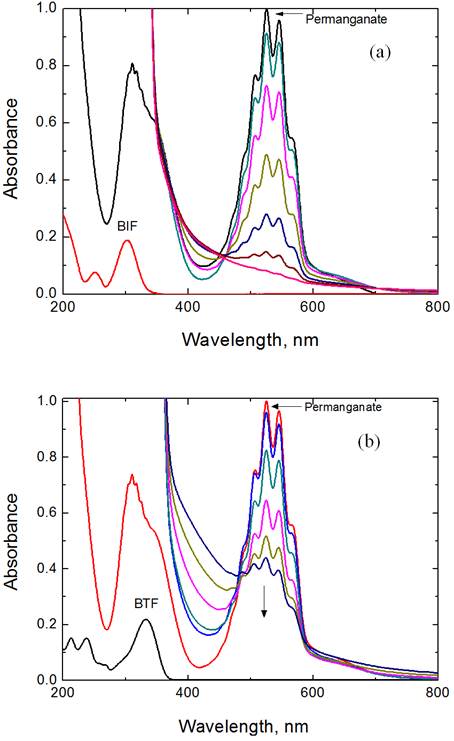
Figure 1. Time-resolved spectra in the oxidation of: (a) BIF, and (b) BTF by permanganate ion in diluted sulfuric acid medium; [S] = 6.0 x 10-3, [MnO4−] = 4.0 x 10−4, [H+] = 5.0 x 10-2, and I = 0.2 mol dm−3 at 25°C. Scanning time interval = 1 min.
3. Result and Discussion
3.1. Reactions Stoichiometry and Products Identification
Various sets of the reactions mixtures containing different initial concentrations of the reactants at constant [H+] and ionic strength were equilibrated in dark. The unreacted permanganate concentration was estimated by both titrimetric and spectrophotometric techniques. The results indicate
consumption of two moles permanganate ions for five moles of BIF or BTF to yield the final oxidation products as shown in the following equation,
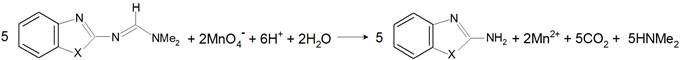
where X = NH for benzimidazolyl-formamidine (BIF) and X = S for benzthiazolyl-formamidine (BTF).
The above stoichiometric equation is consistent with the results of products identification, where the final oxidation products of BIF and BTF were identified by both spectroscopic and chemical tools as described elsewhere [16, 32, 33] as 2-aminobenzimidazole and 2-aminobenzthiazole, respectively, in addition to dimethylamine and carbon dioxide. Dimethylamine was identified by spot tests [33] and carbon dioxide by lime water.
3.2. Reaction–Time Curves
The reactions–time curves throughout the entire range of reactions, illustrated in Figure 2, were found to consist of two stages. The initial stage was found to be slow (induction period), followed by an increase in the oxidation rates over longer times (auto-acceleration period). As the reactions are of catalytic nature, it obeys the rate expression: (At− A∞) = Boe−kst+ Poe−kft where ks and kf are the observed first order rate constants for the induction and auto-acceleration periods, respectively, At and A∞ are the absorbance at times t and infinity; while Bo and Po represent the absorbance change for the slow and fast reacting species, respectively. The rate constants were obtained by drawing a straight line through the slow-time linear portion (ks) of the first order plot and extrapolating the time back to zero time (Bo). The rate of oxidation for the auto-acceleration period, kf, was obtained from plots of the form: ln[(At−A∞)−(A∞ −At)] − t where the quantity (At−A∞) represents the experimental point and (A∞ −At) is the extrapolated one at time t [34]. The values of ks and kf were calculated by the least-squares method and were reproducible to within 3-4%. The orders of reactions with respect to the reactants were determined from the slopes of the log ks versus log (Conc.) plots by varying the concentrations of the reductants and acid, in turn, while keeping other conditions constant.
3.3. Dependence of the Oxidation Rates on [MnO4-]
The effect of permanganate ion oxidant was studied by varying its initial concentration in the range of (1.0 – 8.0) × 10−4 mol dm−3 at constant concentrations of both reductants and sulfuric acid, and at fixed ionic strength and temperature. The values of ks were found to be almost constant, Table 1, indicating first order dependence with respect to [MnO4-].
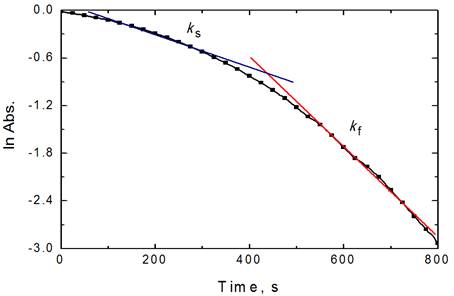
Figure 2. Reaction-time curve in the oxidation of BTF by permanganate ion in diluted sulfuric acid medium.[MnO4−] = 4.0 x 10−4, [BTF] = 6.0 x 10-3, [H+] = 5.0 x 10-2, and I = 0.2 mol dm−3 at 25 °C.
3.4. Dependence of the Oxidation Rates on [S]
The reductants BIF and BTF (abbreviated by S) were varied in the concentration range (2.0 – 10.0) × 10−3 mol dm−3, at constant concentrations of permanganate ion and acid, and at constant ionic strength and temperature. It was observed that ks increased with increasing reductants concentrations as listed in Table 1. The plots of ks versus [BIF] and [BTF] were linear with positive intercepts as shown in Figure 3 confirming the fractional-first order dependences with respect to reductants concentrations.
3.5. Dependence of the Oxidation Rates on [H+]
In order to clarify the effect of [H+] on the oxidation rates to elucidate the oxidation mechanism, kinetic runs were performed at different hydrogen ion concentrations in the range of (1.0 – 10.0) x 10-2 mol dm−3 at constant other conditions. Increasing acid concentration was found to accelerate the oxidation rates, suggesting that the oxidation reactions were acid-catalyzed. Under our experimental conditions, the plots of log ks versus log [H+] were linear with slopes of 0.71 and 0.79 for BIF and BTF, respectively (Fig. 4) confirming that the orders of the reactions with respect to [H+] were fractional-first.
Table 1. Effect of variation of [MnO4-], [S], [H-] and I on the observed first order rate constants (ks) in the oxidation of BIF and BTF by permanganate ion in diluted sulfuric acid medium.
| 104 [MnO4-] (mol dm-3) | 103 [S] (mol dm-3) | 102 [H+] (mol dm-3) | I (mol dm-3) | 105 ks (s-1) |
| BIF | BTF |
| 1.0 | 6.0 | 5.0 | 0.2 | 377.1 | 196.0 |
| 2.0 | 6.0 | 5.0 | 0.2 | 363.9 | 193.2 |
| 4.0 | 6.0 | 5.0 | 0.2 | 373.8 | 195.4 |
| 6.0 | 6.0 | 5.0 | 0.2 | 381.0 | 188.9 |
| 8.0 | 6.0 | 5.0 | 0.2 | 384.1 | 201.8 |
| 4.0 | 2.0 | 5.0 | 0.2 | 154.9 | 69.4 |
| 4.0 | 4.0 | 5.0 | 0.2 | 275.0 | 145.3 |
| 4.0 | 6.0 | 5.0 | 0.2 | 373.8 | 195.4 |
| 4.0 | 8.0 | 5.0 | 0.2 | 464.3 | 250.1 |
| 4.0 | 10.0 | 5.0 | 0.2 | 559.7 | 297.4 |
| 4.0 | 6.0 | 1.0 | 0.2 | 135.6 | 58.9 |
| 4.0 | 6.0 | 3.0 | 0.2 | 260.3 | 134.1 |
| 4.0 | 6.0 | 5.0 | 0.2 | 373.8 | 195.4 |
| 4.0 | 6.0 | 7.0 | 0.2 | 478.6 | 269.8 |
| 4.0 | 6.0 | 9.0 | 0.2 | 593.9 | 335.5 |
| 4.0 | 6.0 | 5.0 | 0.2 | 373.8 | 195.4 |
| 4.0 | 6.0 | 5.0 | 0.3 | 383.1 | 191.6 |
| 4.0 | 6.0 | 5.0 | 0.4 | 366.0 | 201.7 |
| 4.0 | 6.0 | 5.0 | 0.5 | 371.4 | 198.3 |
| 4.0 | 6.0 | 5.0 | 0.6 | 364.3 | 183.7 |
Experimental error ±3%
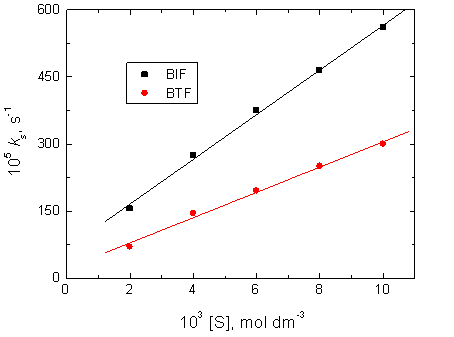
Figure 3. Effect of reductants concentration, [S], on the observed first order rate constant of the slow stage (ks) in the oxidation of BIF and BTF by permanganate ion in diluted sulfuric acid medium. [MnO4−] = 4.0 ×10−4, [H+] = 5.0 x 10-2 and I = 0.2 mol dm−3 at 25°C.
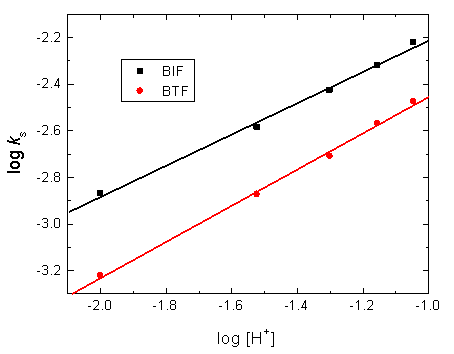
Figure 4. Plots of log ks versus log [H+] in the oxidation of BIF and BTF by permanganate ion in diluted sulfuric acid medium. [MnO4−] = 4.0 ×10−4, [S] = 6.0 x 10-3 and I = 0.2 mol dm−3 at 25°C.
3.6. Effect of Ionic Strength and Dielectric Constant
The ionic strength was varied from 0.2 to 0.6 mol dm−3 using sodium sulfate at constant concentrations of other reactants and temperature. Increasing the ionic strength had a negligible effect on the oxidation rates as listed in Table 1. Furthermore, at constant concentrations of reactants and with other conditions constant, the concentration of acetic acid was varied from 0% to 40% (v/v) in the reactions media. Changing the dielectric constant of the media did not have any significant effect on the oxidation rates.
3.7. Dependence of the Oxidation Rates on [MnII]
The effect of manganese(II) ion, as one of the oxidation products, on the oxidation rates was examined by addition of
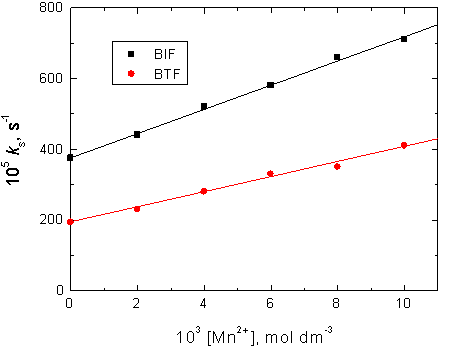
Figure 5. Effect of added [MnII] on the rates of oxidation of BIF and BTF by permanganate ion in diluted sulfuric acid medium. [MnO4−] = 4.0 x10−4, [S] = 6.0 x 10−3, [H+] = 5.0 x 10-2 and I = 0.2 mol dm−3 at 25°C.
different concentrations of MnIISO4 in the range of at fixed other conditions. The experimental observations indicated that the oxidation rates increased with increasing [Mn2+] with a complete disappearance of the induction period and the orders with respect to [Mn2+] were less than unity, as shown in Figure 5.
3.8. Effect of Temperature
The rates of oxidation reactions of both organic reductants were measured at five different temperatures in the range of 15 - 35°C at constant concentrations of the reactants and other conditions being constant. The results indicate that the rate constants increased with rise in temperature. The activation parameters of the second order rate constant (k2) are calculated using Eyring and Arrhenius equations and are listed in Table 2.
Table 2. Activation parameters of the second order rate constants (k2) in the oxidation of BIF and BTF by permanganate ion in diluted sulfuric acid medium. [MnO4−] = 4.0 x10−4, [S] = 6.0 x 10−3, [H+] = 5.0 x 10-2 and I = 0.2 mol dm−3 at 25°C.
| Organic Reductant | DS≠ J mol-1K-1 | DH≠ kJ mol-1 | DG≠298 kJ mol-1 | Ea≠ kJ mol-1 |
| BIF | -88.91 | 29.05 | 55.45 | 31.77 |
| BTF | -107.03 | 32.71 | 64.60 | 35.01 |
3.9. Polymerization Tests
To check the involvement of free radicals in the present oxidation reactions, the reactions mixtures were mixed with known quantities of acrylonitrile monomer and kept for 6 h under nitrogen. On dilution with methanol, white precipitates were formed, indicating the participation of free radicals in the oxidation reactions. Blank experiments carried out with either MnO4− or reductants alone with acrylonitrile did not induce polymerization under the same experimental conditions.
3.10. Reaction Mechanism
Permanganate ion in various media provides excellent results when used in oxidation processes. Manganese(VII) is reduced to MnII during the oxidation reactions via many manganese species having different oxidation states such as MnVI, MnV, MnIV and MnIII. The appearance of these intermediate oxidation states depends on the reaction conditions and the type of substrate. It was reported [35] that reduction of permanganate ion in an acidic medium gives either MnIV or MnII; the reduction potential of the MnVII/MnIV couple is 1.695 V and that of the MnVII/MnII couple is 1.51 V. In strongly acidic media, MnVII is reduced, ultimately forming MnII, but the species that has the main role as a potential oxidant depends on the nature of the substrate and the pH of the medium [36]. Oxidation reactions involving permanganate ion as an oxidant are suggested [13-24] to proceed throughout intermediate complex formation between oxidant and reductant. The formation of manganate(VI) and/or hypomanganate(V) short-lived intermediates may be confirmed by the change in the color of the solution mixture as the reaction proceeded from purple-pink, MnVII, to blue, MnV, to green, MnVI. The failure to detect MnV, absence of an absorption maximum around λ = 700 nm, may be interpreted by its extreme short lifetime and undergoing a rapid disproportionation [21, 37]. Also, the enhancement of the reaction rate with increasing acid concentration and the chemistry of potassium permanganate suggest [38, 39] formation of a more powerful oxidant, namely permanganic acid, by the equilibrium:
MnO4- + H+ 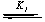 HMnO4
HMnO4
where K1 is the protonation constant of permanganate ion. The protonation of permanganate ion shifts the MnVII/MnVI couple to a more positive value (+1.3 V), which makes HMnO4 a stronger oxidizing agent than MnO4- [39].
The present reactions between the investigated organic reductants (BIF and BTF) and permanganate ion in diluted sulfuric acid medium have a stoichiometry of 5: 2 (reductant: permanganate) with a first order dependence on [MnO4-] and fractional-first order dependences with respect to both [H+] and [reductant]. The fractional-first order dependences on [H+] suggests protonation of permanganate ion to form a more powerful oxidant (permanganic acid) in the first step of the suggested mechanism. Also, the less-than-unit order dependences with respect to reductants concentrations suggests formation of intermediate complexes between reductants and permanganate ion in the pre-equilibrium step. The kinetic evidence for complexes formation was obtained from the plots of 1/ks versus 1/[S] which they were found to be linear with positive intercepts on 1/[S] axes as shown in Figure 6, similar to the well-known Michaelis–Menten mechanism for enzyme–substrate reactions [40]. On the other hand, the negligible effects of both ionic strength and dielectric constant of the reactions media on the oxidation rates indicate that the reactions occur between two neutral molecules [41, 42], i.e. between organic reductant and permanganic acid.
In view of the above arguments, the following reactions mechanism, Scheme 1, can be suggested. The mechanism involves attack of the powerful permanganic acid on one mole of organic reductant in a pre-equilibrium step to give an intermediate complex (C). The cleavage of such complex leads to formation of a free radical derived from organic reductant and an intermediate MnVI species. Such intermediate is rapidly attacked by manganate(VI) ion to yield the corresponding secondary alcohol, as an intermediate product, and MnV species. The intermediate product is rapidly hydrolyzed to give the final oxidation products. In a further fast step, the intermediate MnV being very active and unstable reacts with another organic reductant to yield again the final oxidation products and an intermediate MnIII species. This step is followed by reactions between two molecules of organic reductants and one molecule of permanganic acid giving other oxidation products and another MnIII species. The last step is the reaction between the fifth molecule of organic reductant and two produced MnIII species leading to formation of the oxidation products of organic reductant and MnII as the final oxidation product of permanganate, satisfying the observed reactions stoichiometry.
On the other hand, increasing the oxidation rates and disappearance of the induction period upon increasing added MnII ion may suggest that MnIII and/or MnIV are the sole oxidants throughout the auto-acceleration final stage. However, it is difficult to decide whether MnIII or MnIV was the reactive species in the auto-acceleration period. However, in similar redox reactions involving MnO4− as an oxidant, the continuous increase in the oxidation rate with increasing added MnII in addition to formation of free radicals suggested that MnIII is the more predominant ion [43].
where X = NH for BIF and X = S for BTF
Scheme 1. Mechanism of oxidation of BIF and BTF by permanganate ion in diluted sulfuric acid medium.
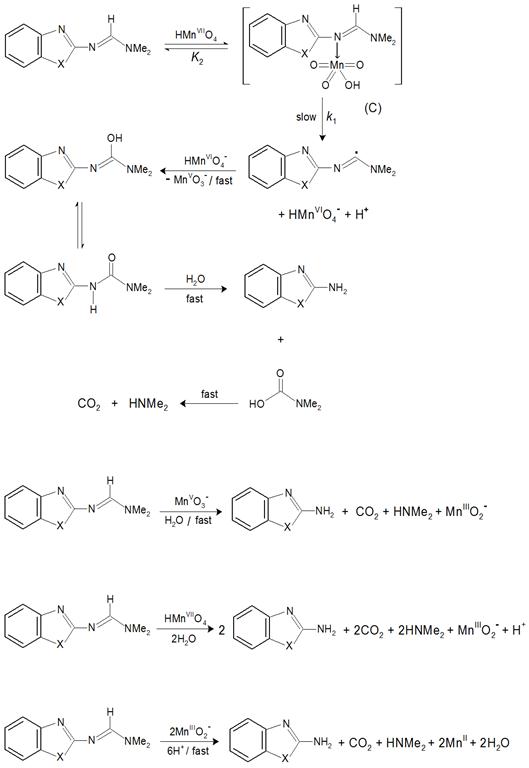
According to the suggested mechanism, the relationship between the oxidation rate and the oxidant, [MnO4-], reductant, [S], and hydrogen ion, [H+], concentrations was derived (see Appendix A) and gave the following equation:
Rate (1)
Under pseudo-first-order conditions, the rate law can be expressed as:
Rate=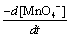 = ks[MnO4-] (2)
= ks[MnO4-] (2)
Comparing Eqs. (1) and (2) and rearrangement gives the following relationship:
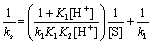 (3)
(3)
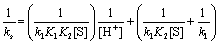 (4)
(4)
According to Eqs. (3) and (4), other conditions being constant, plots of 1/ks versus 1/[S] at constant [H+] and 1/ks versus 1/[H+] at constant [S] should be linear with positive intercepts on the 1/ks axes and are indeed found to be so as shown in Figures 6 and 7, respectively. The slopes and intercepts of such plots lead to calculation of the values of k1, K1 and K2 as listed in Table 3.
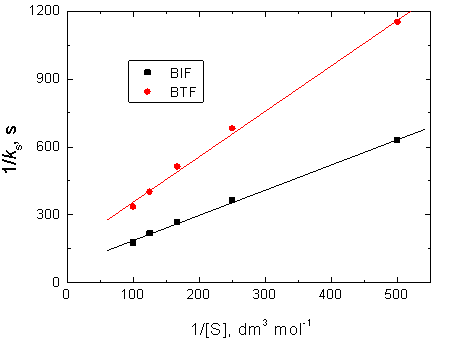
Figure 6. Verification of equation (3) in the oxidation of BIF and BTF by permanganate ion in diluted sulfuric acid medium. [MnO4−] = 4.0 x10−4, [H+] = 5.0 x 10-2 and I = 0.2 mol dm−3 at 25°C.
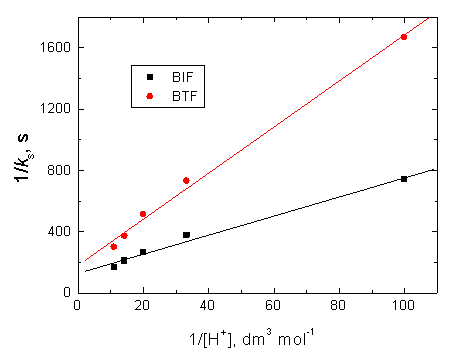
Figure 7. Verification of equation (4) in the oxidation of BIF and BTF by permanganate ion in diluted sulfuric acid medium. [MnO4−] = 4.0 x10−4, [S] = 6.0 x 10-3 and I = 0.2 mol dm−3 at 25°C.
Table 3. Values of k1, K1 and K2 in the oxidation of BIF and BTF by permanganate ion in diluted sulfuric acid medium. [MnO4−] = 4.0 x10−4, [S] = 6.0 x 10-3, [H+] = 5.0 x 10-2 and I = 0.2 mol dm−3 at 25°C.
| Organic Reductant | 103 k s-1 | K1 dm3 mol-1 | K2 dm3 mol-1 |
| BIF | 13.31 | 13.78 | 145.63 |
| BTF | 8.11 | 7.08 | 272.14 |
The activation parameters listed in Table 2 may be interpreted as follows. The obtained large negative values of DS≠ suggest compactness of the formed complexes and such complexes are more ordered than the reactants due to loss of degrees of freedom [44, 45]. Also, the obtained values of DS≠ are within the range of radical reactions. The positive values of both DH≠ and DG≠ confirm endothermic formation of the intermediate complexes and their non-spontaneities, respectively.
4. Conclusion
The kinetics of oxidation of N,N-dimethyl-N’-(1H-benzimidazol-2-yl) formamidine (BIF) and N,N-dimethyl-N’-(benzthiazol-2-yl) formamidine (BTF) by permanganate ion in diluted sulfuric acid medium has been investigated. The final oxidation products of BIF and BTF were identified as 2-aminobenzimidazole and 2-aminobenzthiazole, respectively, in addition to dimethylamine and carbon dioxide. Under comparable experimental conditions, the oxidation rate of BIF was higher than that of BTF. A plausible reactions mechanism has been suggested and the activation parameters have been computed and discussed.
Appendix A
According to the suggested mechanism,
Rate = k1[C] (A1)
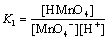 (A2)
(A2)
Therefore,
[HMnO4] = K1[MnO4−][H+] (A3)
From reaction (4),
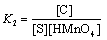 (A4)
(A4)
Therefore,
[C] = K2[S][HMnO4] (A5)
Substituting Eq. (A3) into Eq. (A5) leads to
[C] = K1K2[S][H+][MnO4−] (A6)
Substituting Eq. (A6) into Eq. (A1) yields
Rate = k1K1K2[S][H+][MnO4−] (A7)
The total concentration of the reductants (S) is given by
[S]T = [S]F + [C] (A8)
where [S]T and [S]F stand for total and free concentrations of the substrate.
Substituting Eq. (A6) into Eq. (A8) gives
[S]T = [S]F + K1K2[S]F[H+][MnO4−] (A9)
[S]T = [S]F (1+ K1K2[H+][MnO4−]) (A10)
Therefore,
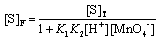 (A11)
(A11)
Similarly,
[MnO4−]T = [MnO4−]F + [HMnO4] + [C] (A12)
Substituting Eqs. (A3) and (A6) into Eq. (A12) gives
[MnO4−]T = [MnO4−]F + K1[MnO4−]F[H+] +
K1K2[S][H+][MnO4−]F (A13)
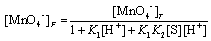 (A14)
(A14)
and
[H+]T = [H+]F + [HMnO4] (A15)
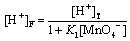 (A16)
(A16)
Substituting Eqs. (A11), (A14), and (A16) into Eq. (A7) (and omitting the subscripts "T" and "F") we get
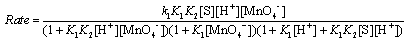 (A17)
(A17)
In view of the low concentration of [MnO4−] used, the first and second terms in the denominator of Eq. (A17) both approximate to unity. Therefore, Eq. (17) becomes
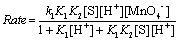 (A18)
(A18)
Under pseudo-first-order conditions, the rate law can be expressed as
Rate = 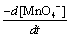 = ks[MnO4−] (A19)
= ks[MnO4−] (A19)
Comparing Eqs. (A18) and (A19), the following relationship is obtained
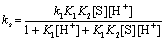 (A20)
(A20)
and with rearrangement it becomes
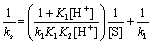 (A21)
(A21)
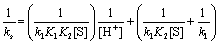 (A22)
(A22)
References
- Keri RS, Patil MR, Patil SA, Budagumpi S (2015) A comprehensive review in current developments of benzothiazole based molecules in medicinal chemistry. Eur. J. Med. Chem., 89: 207-251.
- Abdul Rouf CT (2014) Bioactivethiazole and benzothiazole derivatives. Eur. J. Med. Chem., 1-17.
- Hisamoddin SZK, Priyanka S, Yogesh SP, Patel Nilam PU (2014) Benzothiazole the molecule of diverse biological activities. Pharma Sci. Monitor, 5:207-225.
- Shi B, Chen R, Huang Y (2004) Synthesis of N-(benzothiazol-2-yl-aminodialkyl)-thiophosphate. Gaodeng Xuexiao Huaxue Xuebao, 25:1458–1460.
- Huang ST, Hsei LJ, Chen C (2006) Synthesis and anticancer evaluation of bis(-benzimidazoles), bis(benzoxazoles), and benzothiazoles, Bioorg. Med. Chem., 14:6106-6119.
- Kamal A, Kumar BA, Arifuddin M, Dastidar SG (2006) Synthesis and biological activity of new 4β-N-heteroaryl analogues of podophyllo. Lett. Drug Design. Dis., 3:205–209.
- Yongseog Ch, Young-Kook Sh, Chang-Guo Z, Sungduck L, Hoon Ch (2004) Synthesis and evaluation of antitumor activity of 2- and 6-[(1,3-benzothiazol-2-yl) aminomethyl]-5,8-dimethoxy-1,4-naphthoquinone derivatives, Arch. Pharm. Res. 27:893–890.
- Singh SP, Segal S (1988) Study of fungicidal activities of some benzothiazoles, Ind. J. Chem., 27B: 941-943.
- Suresh CH, Rao JH, Jayaveera KN, Subudhi SK (2013) Synthesis and anthelmintic activity of 3-(2-hydrozino benzothiazole)-substituted indole-2-one. Int. J. Pharm., 2: 257-261.
- Akhtar T, Hameed S, Al-Masoudi N, Loddo R, Colla P (2008) In vitro antitumor and antiviral activities of new benzothiazole and 1,3,4-oxadiazole-2-thione derivatives. Acta Pharm., 58: 135-149.
- Abdel-Rahman HM, Morsy MA (2007) Novel benzothiazolyl urea and thiourea derivatives with potential cytotoxic and antimicrobial activities. J. Enz. Inh. Med. Chem., 22:57–64.
- Abdel-Zaher A, Elassar A (2015) Synthesis of benzoazolyl-N,N-dimethylformamidines: complexation and biological activity. Eur. Int. J. Sci. Technol., 4: 88-99.
- Fawzy A, Ashour SS, Musleh MA (2014) Base-catalyzed oxidation of L-asparagine by alkaline permanganate and the effect of alkali-metal ion catalysts: kinetics and mechanistic approach, React. Kinet. Mech. Catal. 111:443-460.
- Fawzy A, Shaaban MR (2014) Kinetic and mechanistic investigations on the oxidation of N’-heteroaryl unsymmetrical formamidines by permanganate in aqueous alkaline medium. Transition Met. Chem. 39: 379-386.
- Fawzy A, Zaafarany IA, Alfahemi J, Tirkistani FA (2015) Base-catalyzed oxidation of aminotriazole derivative by permanganate ion in aqueous alkaline medium: a kinetic study. Int. J. Inn. Res. Sci. Eng. Tech., 4: 6802-6814.
- Fawzy A, Zaafarany, Althagafi I, Al-Bonayan A, Aljiffrey F (2016)Kinetic and mechanism of oxidation of benzazolyl-formamidines by permanganate in alkaline medium. Am. J. Appl. Chem. 4: 50-58.
- Asghar BH, Fawzy A (2014) Kinetic, mechanistic, and spectroscopic studies of permanganate oxidation of azinyl-formamidines in acidic medium, with autocatalytic behavior of manganese(II). J. Saudi Chem. Soc., in press.
- Fawzy A, Ashour SS, Musleh MA (2014) Kinetics and mechanism of oxidation of L-histidine by permanganate ions in sulfuric acid medium. Int. J. Chem. Kinet. 46: 370-381.
- Simandi KI, Jaky M, Schelly ZA (1984) Short-lived manganate(VI) and manganate(V) intermediates in the permanganate oxidation of sulfite ion, J. Am. Chem. Soc., 106: 6866-6867.
- Simandi LI, Jaky M, Savage CR, Schelly ZA (1985) Kinetics and mechanism of the permanganate ion oxidation of sulfite in alkaline solutions. The nature of short-lived Intermediates, J. Am. Chem. Soc. 107: 4220-4224.
- Ahmed GA, Fawzy A, Hassan RM (2007) Spectrophotometric evidence for the formation of short-lived hypomanganate(V) and manganate(VI) transient species during the oxidation of K-carrageenan by alkaline permanganate. Carbohydr. Res. 342: 1382-1386.
- Zaafarany IA, Fawzy A, Ahmed GA, Ibrahim SA, Hassan RM, Takagi HD (2010) Further evidence for detection of short-lived transient hypomanganate(V) and manganate(VI) intermediatesduring oxidation of some sulfated polysaccharides by alkaline permanganate using conventional spectrophotometeric techniques. Carbohydr. Res. 345: 1588-1593.
- Hassan RM, Fawzy A, Alarifi A, Ahmed GA, Zaafarany IA, Takagi HD (2011) Base-catalyzed oxidationof some sulfated macromolecules: kinetics and mechanism of formation of intermediate complexes of short-lived manganate (VI) and/or hypomanganate (V) during oxidation of iota- and lambda-carrageenan polysaccharides by alkaline permanganate. J. Mol. Catal. A, 335: 38-45.
- Hassan RM, Dahy A, Ibrahim S, Zaafarany IA, Fawzy A (2012) Oxidation of some macromolecules. Kinetics and mechanism of oxidation of methyl cellulose polysaccharide by permanganate ion in acid perchlorate solutions. Ind. Eng. Chem. Res. 51: 5424–5432.
- Gardner KA, Kuehnert LL, Mayer JM (1997) Hydrogen atom abstraction by permanganate: oxidations of arylalkanes in organic solvents. Inorg. Chem. 36: 2069-2078.
- Kini AK, Farokhi SA, Nandibewoor ST (2002) A comparative study of ruthenium(III) catalysed oxidation of L-leucine and L-isoleucine by alkaline permanganate. A kinetic and mechanistic approach. Transition Met. Chem. 27: 532–540.
- Halligudi LL, Desai SM, Mavalangi AK, Nandibewoor ST (2000) Kinetics of the oxidative degradation of rac-serine by aqueous alkaline permanganate. Monatsh. Chem. 131: 321–332.
- Verma RS, Reddy JM, Shastry VR (1974) Kinetic study of homogeneous acid-catalyzed oxidation of certain amino-acids by potassium permanganate in moderately concentrated acidic media. J. Chem. Soc. Perkin Trans. 124: 469–473.
- Mohanty B, Behera J, Acharya S, Mohanty P, Pantaik AK (2013) Metal ion catalyzed oxidation of L-lysine by alkaline permanganate Ion-A kinetic and mechanistic approach. Chem. Sci. Trans. 2: 51–60.
- Jose TP, Nandibewoor ST, Tuwar SM (2005) Mechanism of oxidation of L-histidine by heptavalent manganese in alkaline medium. E-J. Chem., 2: 75-85.
- Vogel AI (1978) A text book of quantitative inorganic analysis. 4th ed, pp. 352, ELBS and Longman, New York.
- Vogel AI (1973) Text book of practical organic chemistry including quantitative organic analysis, 3rd ed, pp. 332, ELBS and Longman, New York.
- Feigl F (1975) Spot tests in organic analysis, pp. 195, Elsevier, New York.
- Frost AA, Person RG (1973) Kinetics and Mechanism, pp. 147, Wiley Eastern, New Delhi.
- Day MC, Selbin J (1985) Theoretical Inorganic Chemistry, Reinhold Publishing Corporation, New York, pp. 344.
- Stewart R (1965) Oxidation in Organic Chemistry, Part A (ed.) Wiberg KB, New York, Academic Press.
- Zimmerman CL. Ph D. (1949) Thesis University of Chicago.
- Bailey N, Carrington A, Lott T, Symons MCRJ (1960) Structure and reactivity of the oxyanions of transition metals. Part VIII. Acidities and spectra of protonated oxyanions. J. Chem. Soc. 290-297.
- Carrington A, Symons MCRJ (1963) Structure and reactivity of the oxyanions of transition metals. Chem. Rev. 63:443-460.
- Michaelis L, Menten ML (1913) The kinetics of invertase action. Biochem. Z. 49: 333–369.
- Amis ES (1966) Solvent effect on reaction rates and mechanism, pp. 28, Academic Press, New York.
- Laidler K (1965) Chemical Kinetics. Pp. 123, McGraw-Hill, New York.
- Waters WA (1958) Rev. Chem. Soc. 12, 277-281; Radhakrisshnamurti PS, Rao MD (1977) Ind. J. Chem. 15A: 524–527.
- Hicks KW, Toppen DL, Linck RG (1972) Inner-sphere electron-transfer reactions of vanadium(II) with azidoamine complexes of cobalt(III). Inorg. Chem. 11: 310-315.
- Weissberger A (1974) In Investigation of rates and mechanism of reactions in techniques of chemistry, (New York: John Wiley & Sons), pp. 421.

 (Fawzy A.)
(Fawzy A.)