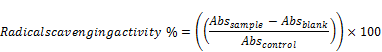Influence of Extraction Solvents and Phytochemical Analysis in the Evaluation of in-vitro Antioxidant Activity of Saudi Arabian Olive Leaves Extract
Bassem Yousef Sheikh1, *, Sami Gabr2
1Scientific Miracles of Prophetic Medicine, Faculty of Medicine, Taibah University, Al-Madinah Al-Munawarah, Saudi Arabia
2King Saud University, Riyadh, Saudi Arabia
Email address

(B. Y. Sheikh)
*Corresponding author
Citation
Bassem Yousef Sheikh, Sami Gabr. Influence of Extraction Solvents and Phytochemical Analysis in the Evaluation of in-vitro Antioxidant Activity of Saudi Arabian Olive Leaves Extract. American Journal of Chemistry and Application. Vol. 3, No. 2, 2016, pp. 6-12.
Abstract
The geographical origin of plant significantly affects its phytochemicals constituents. This study evaluated the antioxidants activities of Saudi Arabian olive leave extracts (OLEs) using different extraction solvents. Deionized distilled water (ddH2O), 80 methanol (80 MeOH), 70 ethanol (70 EtOH), and 80 acetone were used as solvents. The antioxidant activities of the OLEs were observed using DPPH, β-carotene bleaching, and antioxidant capacity standard assays. Highest total phenol, flavonoid and vitamin c contents of the OLEs was observed to be 26.15 mg TAE/g dry weight powder (DW), 25.67 mg catechin/g DW and 198.7mg ascorbic acid/g DW, respectively. GC-MS analysis revealed the presence of bioactive compounds such as oleuropein, caffeic acid, vanillin, rutin, glucosides, quercetin, luteolin, apigenin, and traces of chryseriol. The antioxidant activities of OLEs showed variation according to the solvent used. Higher radical scavenging activity (91.2 µg/ml) was observed in 80 MeOH solvent. There is a significant (p<0.05) positive correlation between the total phenolics contents and extract’s antioxidant activities. The data indicated that OLEs is a promising source of potential antioxidants and may serve as alternative therapeutic agent in the pathogenesis of some diseases.
Keywords
Antioxidants, Alternative Medicine, DPPH, Free Radicals, Olive, Phytochemicals, Phytotherapy
1. Introduction
Free radicals are unstable oxygen molecules or atoms with unpaired electrons referred to as reactive oxygen species (ROS). The oxygen molecules tend to oxidize other compounds, and this oxidation causes an imbalance between formation and neutralization of prooxidants that seek stability through electron pairing with biochemical macromolecules such as proteins, lipids and DNA. Thus, incurring oxidative stress related protein and DNA damage, in addition to lipid peroxidation in the physiological system [1-4]. The oxidative stress particularly that of ROS and lipid peroxidation is known to be among the factors that influences the pathogenesis of diseases. Continuous production of these ROS in living organisms results in the activation of various signaling pathways [5]. Example, it could lead to the activation of angiogenesis by oxidation of reactive cysteine residues on specific target molecules such as redox-sensitive transcription factors and genes, NF-κB, hypoxia-inducible factors (HIFs), VEGF, matrix metalloproteinases (MMPs), COX-2 and inducible nitric oxide synthase (iNOS). Additionally, ROS generation is demonstrated to play a key role in the inflammation process both in vitro and in vivo [6,7].
Therefore, any chemical compound able to reduce the oxidation of other molecules or donate electron to ROS and convert it to harmless molecule is said to possess anti-oxidative power. Thus antioxidants are chemical compounds that neutralize these free radicals by pairing or donating an electron to the free radical changing them from harmful unstable to non-harmful stable molecules. Thence, the effectiveness of a therapeutic chemical in the management of disease is partly owed to its ability to inhibit the production or scavenge the produced ROS.
Although, chemically synthesized antioxidant compounds such as butylated hydroxyl‑toluene and that of hydroxyl-anisole have been in used for several decades, their potential usage is currently questionable due to some reported carcinogenic potency [8]. Hence, the urgent need for alternative antioxidants that are effective and have no or minimal side effects.
Phytochemicals have been used against several disease and ailments since times immemorial [9]. The ability of certain phyto-chemical extracts to inhibit or delay the oxidation of other molecules by suppressing the initiation or propagation of ROS have made them to be an attractive alternative in complementary medicine [10-12]. Such kind of naturally occurring antioxidant chemicals were reported to composed of phenolic compounds such as flavonoids, isoflavones, flavones, anthocyanins, coumarins, lignans, catechins and isocatechins [13], nitrogen compounds such as alkaloids, chlorophyll derivatives, amines and amino acids, carotenoids as well as ascorbic acid [14].
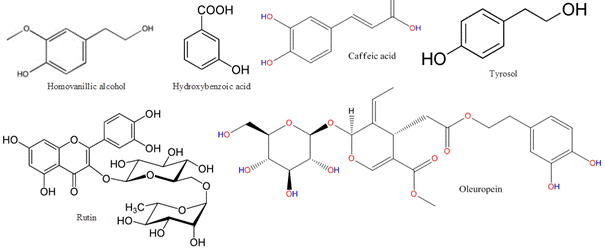
To this length, olive of the family Oleaceae, its oil and leaves have been widely used in Eastern cultures as alternative therapies, food and in religious gathering [15]. Previous studies on the phyto-chemical analysis of olive revealed the presences of several triglycerides; phenolic compounds such as hydroxytyrosol, tyrosol, caffeic acid, p‑hydroxyphenylacetic acid and homovanillic acid as well as several flavonoid esters [16,17]. Most of these phytochemicals compounds are known to exhibit antioxidant properties.
Various extraction techniques, as well as different geographical origin of the olive leaves, result in some differences in the chemical composition of the extracts. So, the present study evaluated the phytochemicals constituents of olive leave extracts (OLEs) and the effect of extraction solvent type on antioxidant activities which may justify its medicinal uses. Thus, our study have employed a number of methods such as 2, 2-diphenyl-l-picrylhydrazyl (DPPH), beta-carotene bleaching test, total phenolic, total flavonoids, and vitamin C contents in evaluating the antioxidant activity of the extracted bioactive compounds.
2. Materials and Methods
Olive leaves were purchased from the local spice shop (Othaim Markets) in Riyadh, Saudi Arabia. All the chemicals and reagents used were of analytical grade, and were either purchased from AppliChem or Merck Company.
2.1. Extract Preparation
Four solvents were used for extracting phenolics from leaves: 80% methanol (80 MeOH), 70% ethanol (70 EtOH), 80% acetone and deionized water (ddH2O). One gram of leaves was ground with a mortar and pestle under liquid nitrogen. The grinded leaves were added to a capped centrifuge tube containing 10 mL of solvent. The mixture was allowed to stand in the dark for 24 h. The extract was centrifuged 5000 xg for 10 min, at room temperature, and the supernatants were then filtered using a filter paper (Whatman No. 1). The extracts were concentrated using a rotary evaporator (Heidolphvvl, Germany) under vaccum at operating temperature below 45°C. Thereafter, the extract concentrate was freeze-dried prior to usage.
2.2. Olive Leaves Extract Phytochemical Analysis Using Gas Chromatography Tandem Mass Spectrometry (GC-MS)
GC-MS analysis of the active constituents were performed with GC, using a Hewlett Packard 6890 gas chromatograph equipped with a Hewlett Packard 5973 mass selective detector. The MS was set at electron impact voltage of (70 eV). Mass range was from 35 to 450 m/z. n‑Alkenes were used as reference points in the calculation of the Kovats Indices (K. I.). The machine method was setup according to previous literature [18]. Briefly, aliquot methanolized sample (2 µL) was automatically injected into the GCMS at a split ratio of 1: 10. The injection temperature was set at 300°C. The oven temperature ramping was set as follows: 40°C for 2 min then increased to 150°C at 30°C/min; held at 150°C for 7min then increased to 280°C at 4°C/min; then held at 280°C for 15 min. Helium at 0.76 bar and 14 mL/min was used as the carrier gas. Identification of the bioactive constituents was done by similarity search of the chromatogram relative retention index and mass spectra in the spectral database of National Institute of Standard and Testing (NIST (2009), USA).
2.3. Estimation of Total Phenolics
Total phenolic content of each extract was assayed using Folin-Ciocalteu micro method [19]. Briefly, 20 µl of extract solution or standard solution of tannic acid were mixed with 1.16 ml ddH2O and 100 µl of Folin-Ciocalteu reagent, followed by addition of 300 µl of 20% Na2CO3 solution after 1 min and before 8 min. Subsequently, the mixture was then incubated in a shaking incubator at 40°C for 30 min and its absorbance was measured at 760 nm. Tannic acid was used as a standard for calibration curve. The phenolic content was expressed as mg tannic acid equivalents (TAE) per g of dry weight powder (DW). This was obtained by fitting the absorbance values in the regression equation of the standard calibration curve.
2.4. Estimation of Total Flavonoids
The determination of flavonoids was performed according to the colorimetric assay of Kim et al. [20]. Deionized distilled water (4 mL) was added to 1 mL of olive leaf extract. Then, a 5% sodium nitrite solution (0.3 mL) was added, followed by addition 10% aluminum chloride solution (0.3 mL). Test tubes were incubated at ambient temperature for 5 min, and then 2 mL of 1 M NaOH was added to the mixture. Immediately, the volume was made to 10 mL with ddH2O. The mixture was thoroughly vortexed and the absorbance of the pink color developed was determined at 510 nm. Total flavonoids content was expressed as mg catechin equivalents per g of dry weight powder (mg CE g-1 DW).
2.5. In Vitro Antioxidant Assays
2.5.1. DPPH (1, 1-Diphenyl-2-Picrylhydrazyl) Radical Scavenging Activity
The radical scavenging ability of the total olive leave extract against DPPH was evaluated as previously described [21]. An aliquot sample (125 µl) of 1 mM DPPH in methanol and 30 uL of extract in various concentrations (10, 50, 100, 500, and 1000 µg/mL) were mixed vigorously and the mixture was incubated for 30 min incubation at 25°C, the decrease in absorbance was measured at A = 517 nm against blank. The butylhydroxytoluene was used as standard synthetic antioxidant positive control. The radical scavenging activity was calculated from the equation:
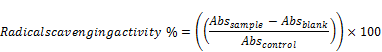
where Abs stands for the absorbance of sample, blank and control, respectively.
2.5.2. Beta-Carotene-Iinoleic Acid Assay
The antioxidant activity of the total olive leave extract was evaluated, using the β‑carotene bleaching method as described by Mothana [22]. Total of 1 mL of a 0.2 mg/mL β-carotene solution in chloroform was added to flasks containing 0.02 mL of linoleic acid and 0.2 mL of Tween-20. The chloroform was removed at 40°C using a rotary evaporator. The resultant mixture was immediately diluted with 100 mL of distilled water and mixed for 1 to 2 min to form an emulsion. A mixture prepared similarly but without β-carotene, was used as a blank. A control containing 0.2 mL of 80 (v/v) methanol instead of extract was also prepared. A 5 mL aliquot of the emulsion was added to a tube containing 0.2 mL of the extract at 1 mg/mL. Rutin (1 mg/mL) was used as a standard. The tubes were incubated in a water bath at 40°C for 2 h. Absorbance was read at 470 nm at 15 min intervals, using a UV–visible spectrophotometer (UV mini-1240, Shimadzu, Japan).
The antioxidant activity was calculated using the following equation:
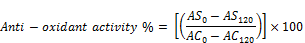
Where AS0, AS120, AC0 and AC120 are absorbance of samples and control at both initial and at 120 min, respectively.
2.5.3. Total Antioxidant Capacity
The total antioxidant capacity was assayed according to lPrieto et al. [23] with minor modifications. An aliquot sample of olive leaves extract or standard was combined with α‑tocopherol reagent solution. The tubes were capped and incubated in a boiling water bath at 95°C for 60-90 min. Samples were cooled to room temperature, the absorbance of each was measured at 695 nm using UV/visible spectrophotometer (Perkin Elmer, Lambda 25, USA). The content of total antioxidant capacity was calculated using a standard graph of α-tocopherol and the results were expressed as vitamin E equivalent (mg/g).
2.6. Determination of Vitamin C Content
The olive extract vitamin C content was analyzed following method reported in literature [24] with slight modifications. To 1 mL of total olive leave extract or standard, 0.1 mL of 2, 4dinitrophenyl hydrazine thiourea copper sulphate (DTC) reagent was added and incubated at 37°C for 3 h. After incubation, 1.25 mL of 85% H2SO4 was added under ice-cold condition. The mixture was kept at room temperature for 30 min. The absorbance was measured at 540 nm using UV/visible spectrophotometer (Perkin Elmer, Lambda 25, USA). The vitamin C content was calculated by referencing to a standard graph of ascorbic acid.
2.7. Statistical Analysis
All experiments were carried out in triplicate. The data were analyzed using analysis of variance (ANOVA) and significant differences among means were determined by Duncan's multiple range test at p<0.05 by the SAS software.
3. Results and Discussions
3.1. Phytochemical Analysis
In this study, preliminary qualitative and quantitative analyses of the extract was performed using gas chromatography mass spectrometry (GCMS). The phytoconstituents of olive leaves extract (OLE) were identified as shown in Table 1. As expected, bioactive compounds such as tyrosol, hydroxytyrosol, oleuropein, benzoic acid, homovanillic acid, rutin, caffeic acid and traces of glucosides were observed in the olive leaves extract (Figure 1). The observed major constituent of the OLE was oleuropein, composing 25.65% of the extract (Table 1). The other identified components in trace amounts were caffeic acid, vanillin, rutin, luteolin-7-0- glucoside, apigenine-7-0-glucoside, quercetin, luteolin, apigenin, and chryseriol.
Among the detected compounds, some were reported to have antioxidant and anticancer activities [25-27]. Previous reports on the olive extract phytochemical analysis have reported the occurrence of similar bioactive compounds [28-31].
3.2. Total Phenolics, Flavonoids and Vitamin C Contents
Phenolic metabolites containing aromatic arene (phenyl) ring with at least one acidic hydroxyl residues attached to it were known to be produced in plants. Literatures have illustrated that flavonoids, tannins, etc. were among the major phenolic contents present in plant extracts [28,31,32]. In particular, olive leaves were shown to contain a high concentration of phenolic compounds [33]. The phenolic radicals were reported to be less reactive and possess lower electron reduction potential than the oxygen radicals [32,34]. Owing to these properties, the phenolic compounds are considered to be excellent radical scavengers. Thus, phenolic compounds have the prospect of scavenging reactive oxygen intermediates without invoking further oxidative reactions. Therefore, it has been among the norms of phytochemical research to evaluate the total phenolic content (TPC) as a measure of ascertaining the antioxidant activity of the extracted bioactive compounds. Studies have linked the bioavailability of some phenolic compounds and their derivatives such as oleuropein, tyrosol and hydroxytyrosol, etc. found in olive leaves extracts (OLEs) to its antioxidant properties [35,36]. The association of the phenolic compounds with the prevention of several diseases has raised interest in OLEs [37].
Hence, the current study have evaluated both the TPC and total flavonoids content (TFC) of the olive leaves extracts (Table 2). Our data have illustrated that the mean TP in olive leaf extracts in terms of mg TAE/g powder (DW) spans from 17.8 to 26.15 mg respectively. Our results showed that methanolic, ethanolic and acetone extracts had the highest amount of phenolic content while ddH2O extract contained the lowest amount, an observation which could be attributed to the solvent polarity index. N comparison to the TPC, the TFC content, expressed as mg CE/g DW, varied from 8.5 to 25.67 mg respectively (Table 2). Similar observations of better phenolics extraction in methanol and ethanol were reported [38,39]. Furthermore, our data agree with those reported in the literature which showed that methanol had better recoveries [40] and is specifically effective in extracting polyphenols [41]. Moreover, our observation was found to be in accord with Siddhuraju and Becker [42] report which stated methanol (80%) and ethanol (70%) to be the best solvents for the extraction of antioxidant compounds from Moringa leaves. In addition, Manian et al. [43] found that the methanol extract of green tea and methanol extract of Ficus racemosa (stem barck) contained relatively higher levels of total phenolics than the other extracts. Furthermore, MeOH 70-80 has produced good yield in extracting hydroxycinnamic derivatives, flavones, flavonols and catechins from fruits, legumes, grape seeds and wine pomace [44].
Study has reported that olive fruits is a good source of vitamins (A, B, C, D, E, and K) and mineral like K, Ca, Mg and P [45]. The vitamin C content also contributes to the antioxidant activity, since vitamin C is a very important and powerful antioxidant that works in aqueous environments of the body. Ascorbic acid, behaves as a vinylogous carboxylic acid, wherein the double bond transmits electron pairs between the hydroxyl and the carbonyl. Ascorbate acts as an antioxidant by being available for energetically favourable oxidation [46]. The high content of vitamin C in OLEs encourages its usage in vivo without being toxic. In this study, vitamin C content in OLE extracts expressed as mg ascorbic acid per gram DW, varied from 165.3 to 198.7 mg respectively. Duncan's multiple range tests revealed the increase in TP, TF and vitamin C contents to be significantly (p<0.01) depended on the solvent type (Table 1). According to these results, the extraction contents followed this order: 80 MeOH> 70 EtOH> 80 acetone> ddH2O. The data obtained showed the significant (p< 0.0 1) importance of 80 MeOH solvent compared to other solvents (Table 1). Thus in this study, we observed that 80 MeOH is the most effective solvent for extracting bioactive compounds from olive leaves.
Table 2. Total phenolic, flavonoids and vitamin C contents of olive leaves using different extraction solvents.
| Extraction Solvent | TPC | TFC | Vitamin C |
| 80 MeOH | 26.2 ± 0.8** | 25.6 ± 2.7** | 198.7 ± 0.9** |
| 70 EtOH | 25.5 ± 0.9** | 20.5 ± 1.6** | 193.2 ± 1.2** |
| 80 acetone | 24.4 ± 0.8** | 16.2 ± 1.3** | 188.1 ± 0.8** |
| ddH2O | 17.8 ± 0.7 | 8.5 ± 0.7 | 165.3 ± 0.5 |
Total phenolic content (TPC) expressed as mg T AE/g powder (dw); Total flavonoids content (TFC) expressed as mg cathechin (CE) /g powder (dw) and Vitamin c content expressed as mg ascorbic acid/g powder (dw): * (p<0.05); ** (p<0.01).
3.3. DPPH Radical Scavenging Activity and β-Carotene Bleaching Assay
The direct and rapid reaction between 2, 2-diphenyl-1-picrylhydrazyl (DPPH) radicals and antioxidant has been utilized as a measure for antioxidant analysis [47]. Therefore, the higher the percentage DPPH radical scavenging of a compound the better the antioxidant activity. In our study also the DPPH was found to agree with TPC and TFC observation, thus, the higher the TPC value the higher the percentage DPPH radical scavenging activity. Using DPPH, the free radical scavenging and antioxidant activities of OLE were reported in Table 3. OLE extract of 80 MeOH solvent showed appreciative higher free radical scavenging activitythatvaried from 15.9 to 91.2 compared to the other extraction solvents tested in the study. The observed higher radical scavenging activity of 80 MeOH sample could be due to the higher TPC in the sample, which translates to the fact that phenolic radicals are less reactive and possess lower electron reduction potential than the oxygen radicals [32,34], thus the substitution of 5, 7, 3′, 4′-hydroxy flavonoids resulted in efficient radical scavenging power [48]. In agreement with this observation, the total antioxidant by β-carotene bleaching test was also found to be higher in 80 MeOH (89.5µg/ml) in comparison to the other solvent analysed in the study (Table 3). This value was found to be nearer to the value obtained using standard Rutin (91.8 µg/ml). This observation has ascertained that OLE exhibited DPPH radical-scavenging activity in a solvent dependent manner.
In general, the obtained data showed a statistical significant correlation between OLE phytoconstituents analyses, i.e. TP, TF, and Vitamin c content and its related biological activities as illustrated in Figure 2. Sepulveda-Jimenez [46], have found that for a same methanolic extract of olive leaves possess higher antiradical activity than those obtained with water. And attributed the higher antioxidant activity of the extract to be due to its phenolic compounds. Thus, the olive leaf extracts rich in antioxidants can be employed as a strategy in the prevention of several chronic human diseases since the antioxidants in their natural matrices are generally assumed to be safe, and their concentration is physiologic.
Table 3. DPPH radical scavenging and β-carotene total antioxidant assays of olive leaves extracts.
| Extracts | Radical scavenging activity (µg/ml) | Total antioxidant (µg/ml) |
| | 10 | 50 | 100 | 500 | 1000 | 1000 |
| 80 MeOH | 15.9 | 24.5 | 68.4 | 72.8 | 91.2 | 89.5 |
| 70 EtOH | 14.8 | 23.9 | 64.8 | 69.5 | 87.9 | 85.2 |
| 80 acetone | 12.9 | 18.9 | 44.6 | 59.9 | 81.3 | 75.4 |
| ddH2O | 10.2 | 12.1 | 22.3 | 34.1 | 69.8 | 62.8 |
| Rutin | | | | | | 91.8 |
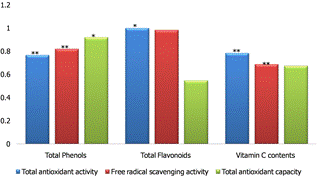
Figure 2. A correlations between total phenols, flavonoids and vitamin C contents in relation to Total antioxidant activity, total antioxidant capacity, and Free Radical scavenging activity. Each value is expressed as mean ± SD, and are significantly different at * p<0.05; ** P<0.01.
4. Conclusion
The antioxidant potential of the olive leaf extracts can be attributed and correlated to its high contents of flavonoids, phenolics and vitamin C that act in an additive and synergistic manner. Although there is an observed significant variation in chemical constituents and biological activities of olive leaf extracts treated with different solvents, the current findings of the present study support that this medicinal plant Olea europaea L. is a promising source of potential antioxidants and may be efficient as alternative therapeutic agent in the pathogenesis of some diseases.
Acknowledgements
This project is supported by Al-Moalim MA Bin Ladin (MABL) chair for Scientific Miracles of Prophetic Medicine, College of Medicine, Taibah University, Saudi Arabia (research grant no. MABL 37/06).
References
- Nagmoti, D.M., D.K. Khatri, P.R. Juvekar, and A.R. Juvekar: Antioxidant activity free radical-scavenging potential of Pithecellobium dulce Benth seed extracts. Free Radicals and Antioxidants. 2 (2), 37-43. (2012)
- Abdelwahab, S.I., B.Y. Sheikh, M.M.E. Taha, C.W. How, R. Abdullah, U. Yagoub, R. El-Sunousi, and E.E. Eid: Thymoquinone-loaded nanostructured lipid carriers: preparation, gastroprotection, in vitro toxicity, and pharmacokinetic properties after extravascular administration. International journal of nanomedicine. 8, 2163. (2013)
- El-Ameen, N.M.H., M.M.E. Taha, S.I. Abdelwahab, A. Khalid, F. Elfatih, M.A. Kamel, and B.Y. Sheikh: Anti-diabetic Properties of Thymoquinone is unassociated with Glycogen Phosphorylase Inhibition. Pharmacognosy Journal. 7 (6). (2015)
- Manal, M.T., I.A. Siddig, E. Rashad, and Y.S. Bassem, et all: Effectiveness of Sidr Honey on the prevention of ethanol-induced gatroulcerogenesis: role of antioxidant and antiapoptotic mechanism.. Pharmacognosy Journal. 17 (3), 157-164. (2015)
- Halabi, M.F. and B.Y. Sheikh: Anti-Proliferative Effect and Phytochemical Analysis of Cymbopogon citratus Extract. BioMed research international. 2014. (2014)
- Kim, Y., B.H. Kim, H. Lee, B. Jeon, Y.S. Lee, M.-J. Kwon, and T.-Y. Kim: Regulation of skin inflammation and angiogenesis by EC-SOD via HIF-1α and NF-κB pathways. Free Radical Biology and Medicine. 51 (11), 1985-1995. (2011)
- Mohamadin, A.M., B. Sheikh, A.A. Abd El-Aal, A.A. Elberry, and F.A. Al-Abbasi: Protective effects of Nigella sativa oil on propoxur-induced toxicity and oxidative stress in rat brain regions. Pesticide Biochemistry and Physiology. 98 (1), 128-134. (2010)
- Ito, N., S. Fukushima, A. Haqlwara, M. Shibata, and T. Ogiso: Carcinogenicity of butylated hydroxyanisole in F344 rats. Journal of the National Cancer Institute. 70 (2), 343-352. (1983)
- Sheikh, B.Y., W.M. Elsaed, A.H. Samman, and A.-M.M.B. Ladin: Ajwa dates as a protective agent against liver toxicity in rat. European Scientific Journal. 10 (7). (2014)
- Maulidiani, M., B.Y. Sheikh, A. Mediani, L.S. Wei, I.S. Ismail, F. Abas, and N.H. Lajis: Differentiation of Nigella sativa seeds from four different origins and their bioactivity correlations based on NMR-metabolomics approach. Phytochemistry Letters. 13, 308-318. (2015)
- Salim, L.Z.A., S. Mohan, R. Othman, S.I. Abdelwahab, B. Kamalidehghan, B.Y. Sheikh, and M.Y. Ibrahim: Thymoquinone induces mitochondria-mediated apoptosis in acute lymphoblastic leukaemia in vitro. Molecules. 18 (9), 11219-11240. (2013)
- Sheikh, B.Y. and A.M. Mohamadin: Thymoquinone a potential therapy for cerebral oxidative stress. Asian Journal of Natural and Applied Sciences. 1 (2), 76-92. (2012)
- Makari, H., N. Haraprasad, H. Patil, and Ravikumar: in vitro antioxidant activity of the hexane and methanolic extracts of Cordia Wallichii and Celastrus paniculata. The Internet J. Aesthetic and Antiaging Medicine. 1, 1-10. (2008)
- Velioglu, Y., G. Mazza, L. Gao, and B. Oomah: Antioxidant activity and total phenolics in selected fruits, vegetables, and grain products. Journal of agricultural and food chemistry. 46 (10), 4113-4117. (1998)
- Hashmi, M.A., A. Khan, M. Hanif, U. Farooq, and S. Perveen: Traditional Uses, Phytochemistry, and Pharmacology of Olea europaea (Olive). Evidence-Based Complementary and Alternative Medicine. 2015. (2015)
- Kiritsakis, A., A. Kanavouras, and K. Kiritsakis: Chemical analysis, quality control and packaging issues of olive oil. European Journal of Lipid Science and Technology. 104 (9-10), 628-638. (2002)
- Stiti, N. and M.-A. Hartmann: Nonsterol triterpenoids as major constituents of Olea europaea. Journal of Lipids. 2012. (2012)
- Gumel, A.M., S.M. Annuar, and T. Heidelberg: Single-step lipase-catalyzed functionalization of medium-chain-length polyhydroxyalkanoates. Journal of Chemical Technology & Biotechnology. 88 (7), 1328–1335. (2013)
- Arabshahi, S. and A. Urooj: Antioxidant properties of various solvent extracts of mulberry (Morus indica L.) leaves. Food Chem. 102, 1233-1240. (2007)
- Kim, D., S. Jeong, and C. Lee: Antioxidant capacity of phenolic phytochemicals from various cultivars of plums. Food Chem. 81, 321-26. (2003)
- Mensor, L.L., F.S. Menezes, G.G. Leitão, A.S. Reis, T.C.d. Santos, C.S. Coube, and S.G. Leitão: Screening of Brazilian plant extracts for antioxidant activity by the use of DPPH free radical method. Phytotherapy research. 15 (2), 127-130. (2001)
- Mothana, R.: Anti-inflammatory, anti-nociceptive and antioxidant activities of the endemic Soqotraen Boswellia elongata Balf f. and Jatropha unicostata Balf. f. in different experimental models. Food Chern Toxicol 49, 2594-9. (2011)
- Prieto, P., M. Pineda, and M. Agulilar: Spectrophotometric quantitation of antioxidant capacity through the formation of a phosphomolybdenum complex: specific application to the determination of vitamin E. Anal Biochem 269 (2), 337-341. (1999)
- Omaye, S., J. Turnbull, and H. Sauberlich: Methods Enzyrnol Selected methods for the determination of ascorbic acid in animal cells, tissues and fluids. 62, 3-11. (1979)
- Khaliq, A., S.D. Ahmad, S.M. Sabir, and A. Khan: Antioxidant activity and inhibitory effect of cultivars of Olive (Olea europaea) against lipid peroxidation in mice liver/Fare karaciğeri lipid peroksidayonuna karşı kültür zeytin’in Olive (Olea europaea) antioksidant ve inhibisyon etkileri. Turkish Journal of Biochemistry. 40 (2), 188-196. (2015)
- Kontogianni, V.G., P. Charisiadis, E. Margianni, F.N. Lamari, I.P. Gerothanassis, and A.G. Tzakos: Olive leaf extracts are a natural source of advanced glycation end product inhibitors. Journal of medicinal food. 16 (9), 817-822. (2013)
- Mateos, R., G. Pereira-Caro, J.R. Bacon, R. Bongaerts, B. Sarriá, L. Bravo, and P.A. Kroon: Anticancer activity of olive oil hydroxytyrosyl acetate in human adenocarcinoma Caco-2 cells. Journal of agricultural and food chemistry. 61 (13), 3264-3269. (2013)
- Angerosa, F., N. d'Alessandro, P. Konstantinou, and L. Di Giacinto: GC-MS evaluation of phenolic compounds in virgin olive oil. Journal of Agricultural and Food Chemistry. 43 (7), 1802-1807. (1995)
- Islamčević Razboršek, M., I. Pavlovič, Ž. Knez, and M. Škerget: GC-MS DETERMINATION OF GLUCOSE AND MANNITOL AFTER OXIMATION AND TRIMETHYLSILYLATION IN OLIVE LEAVES EXTRACTS. Technologica Acta. 8 (1). (2015)
- Tasioula‐margari, M. and O. Okogeri: Isolation and Characterization of Virgin Olive Oil Phenolic Compounds by HPLC/UV and GC‐MS. Journal of Food Science. 66 (4), 530-534. (2001)
- OWEN, R., W. MIER, A. GIACOSA, W. HULL, B. SPIEGELHALDER, and H. BARTSCH: Phenolic compounds and squalene in olive oils: the concentration and antioxidant potential of total phenols, simple phenols, secoiridoids, lignans and squalene. Food and Chemical Toxicology 38, 647-659. (2000)
- Ainsworth, E.A. and K.M. Gillespie: Estimation of total phenolic content and other oxidation substrates in plant tissues using Folin-Ciocalteu reagent. Nat. Protocols. 2 (4), 875-877. (2007)
- Goldsmith, C.D., Q.V. Vuong, C.E. Stathopoulos, P.D. Roach, and C.J. Scarlett: Optimization of the aqueous extraction of phenolic compounds from olive leaves. Antioxidants. 3 (4), 700-712. (2014)
- Gillespie, K.M., J.M. Chae, and E.A. Ainsworth: Rapid measurement of total antioxidant capacity in plants. Nat. Protocols. 2 (4), 867-870. (2007)
- Meirinhos, J., B. Silva, and P. Valentao: Analysis and quantification of flavonoidic compounds from Portuguese olive (Oleae europeae L.) leaf cultivars Nat. Prod. Res. 19, 189-195. (2005)
- Romani, A., N. Mulinacci, P. Pinelli, F. Vincieri, and A. Cimato: Polyphenolic content in five Tuscany cultivars of Olea europaea L.. Journal oj Agricultural and Food Chemistry. 47, 964-967. (1999)
- Cardoso, S., S. Guyot, N. Marnet, J. Lopes-da-Silva, C. Renard, and M. Coimbra: Characterization of phenolic extracts from olive pulp and olive pomace by electrospray mass spectrometry. Journal of the Science of Food and Agriculture. 85, 21-32. (2005)
- Sousa, A., I.C. Ferreira, L. Barros, A. Bento, and J.A. Pereira: Effect of solvent and extraction temperatures on the antioxidant potential of traditional stoned table olives "alcaparras". LWT-Food Science and Technology. 41 (4), 739-745. (2008)
- Lee, O.-H., B.-Y. Lee, J. Lee, H.-B. Lee, J.-Y. Son, C.-S. Park, K. Shetty, and Y.-C. Kim: Assessment of phenolics-enriched extract and fractions of olive leaves and their antioxidant activities. Bioresource technology. 100 (23), 6107-6113. (2009)
- Lihu, Y., J. Yueming, and N. Datta: HPLC analyses of flavanol and phenolic acids in the fresh young shoots of tea (Camellia sinesis) grown in Australia. Food Chem. 84, 253-263. (2004)
- Pinelo, M., M. Rubilar, J. Sineiro, and M. Nunez: Extraction of antioxidant phenolics from almond hulls (Prunus amygdalus) and pine sawdust (Pinus pinaster). Food Chem. 85, 267-273. (2004)
- Siddhuraju, P. and K. Becker: Antioxidant properties of various solvent extracts of total phenolic constituents from three different agroclimatic origins of drumstick tree (Moringa oleifera Lam.) leaves. Journal of agricultural and food chemistry. 51 (8), 2144-2155. (2003)
- Manian, R., N. Anusuya, Siddhuraju, and S. Manian: The antioxidant activity and free radical scavenging potential of two different solvent extracts of Camellia sinensis (L.) O. Kuntz, Ficus bengalensis L. and Ficus racemosa L. Food Chem. 107, 1000-1007. (2008)
- Spigno, G., L. Tramelli, and D. De Faveri: Effect of extraction time, temperature and solvent on concentration and antioxidant activity of grape marc phenolics. J. Food Eng. 81, 200-208. (2007)
- Ibrahim, A. and M. Khalaef, Olive Tree Planting, Protection and Production. 2007, Egypt.
- Sepulveda-Jimenez, F.: Structural chemical and biological aspects of antioxidants for strategies against metal and metalloid exposure. Oxid Med Cell Longev 2, 63-67. (2009)
- Milardović, S., D. Iveković, and B.S. Grabarić: A novel amperometric method for antioxidant activity determination using DPPH free radical. Bioelectrochemistry. 68 (2), 175-180. (2006)
- Tsimogiannis, D.I. and V. Oreopoulou: The contribution of flavonoid C-ring on the DPPH free radical scavenging efficiency. A kinetic approach for the 3′, 4′-hydroxy substituted members. Innovative Food Science & Emerging Technologies. 7 (1–2), 140-146. (2006)

 (B. Y. Sheikh)
(B. Y. Sheikh)