Glycoconjugates Study in Surface Secretions of a Fresh Water Edible Catfish, Clarias gariepinus in Response to Clove Oil Anaesthesia
Ajai Kumar Singh*, Suyash Sawale, Srikant Chavan
Post Graduate Department of Zoology, R. K. Talreja College of Arts, Science & Commerce, Ulhasnagar-3 (MS), India
Email address

(A. K. Singh)
*Corresponding author
Citation
Ajai Kumar Singh, Suyash Sawale, Srikant Chavan. Glycoconjugates Study in Surface Secretions of a Fresh Water Edible Catfish, Clarias gariepinus in Response to Clove Oil Anaesthesia. International Journal of Chemical and Biomedical Science. Vol. 2, No. 5, 2016, pp. 34-41.
Abstract
The present histopathology and histochemistry study investigates the effects of clove oil anaesthesia on gills, dendritic organs and skin of a locally available fresh water edible catfish, Clarias gariepinus. The gills, dendritic organs and skin constitute surface organs in C. gariepinus and remain in direct contact with the surrounding water and provide greater surface area of absorption for any xenobiotics including the clove oil hence selected for study. Clove oil extracted from flower buds, leaves and stems of plant Syzygium aromaticum is recently introduced as an effective anaesthetic agent in fishery sector for fulfilling various needs. C. gariepinus of total length 30 ± 2 cm and total weight 200 ± 5 g were exposed to various concentrations of clove oil (LOBA CHEM. PVT. LTD, MUMBAI, Minimum assay 85%) in the range of 0.07 ml/l to 0.3 ml/l. The important behavioural changes observed in C. gariepinus during clove oil exposures include erratic swimming, bubbling, rubbing to side and bottom walls of glass aquarium and protrusion of head above water surface. Fish however become static and horizontal with ceased opercular movements and loss of equilibrium. Clove oil anaesthesia significantly alters the mucogenic property of all surface organs with secretion of more Alcian Blue pH 2.5 positive mucus and slime on surface. Wear and tear and sloughing of epithelial linings of the gills, disintegration of vascular components, oozing of blood on surface of dendritic organs and lifting of gill epithelium are the other toxic manifestations caused in C. gariepinus due to clove oil anaesthesia.
Keywords
Clove Oil, Anaesthesia, Fish, Surface Organs, Secretions, Behaviour
1. Introduction
Fish are potential bio-indicators hence extensively employed in various toxicology research studies for assessing environmental pollution especially the aquatic pollution [1], [2], [3], [4], [5], [6], [7], [8], [9], [10]. Although understanding about pain and suffering in fish is incomplete, the anaesthetics are often used to provide benefits in relief of pain during various handling and surgical operations. There are several anaesthetics that are in regular practice since many decades. However to find an appropriate anaesthetic in all respects is still a matter of great concern. An anaesthetic is said to be good when it is cost effective, easily available, and easy to handle and takes minimum time for induction and recovery without causing any undue side effects on both the fish and investigator. Clove oil is recently introduced as an anaesthetic in fishery sector for fulfilling various needs including the laboratory research. It is extracted from dried flower buds, leaves and stems of plant Syzygium aromaticum [11]. Clove oil consists of many different compounds with the primary ingredients as (49–87%), β-caryophyllene (4–21%), and eugenyl acetate (0.5–21%). Clove oil is well known for its medicinal values from ancient time as it was/is more commonly used as analgesic and antiseptic. Clove oil is lipophilic and is rapidly absorbed through the skin and because of having this property, it (clove oil) is used in dermal drug delivery mechanisms to enhance drug uptake from the skin [12].
The fresh water air-breathing catfish Clarias gariepinus, the animal model for present study is often available in live condition in local fish market for human consumption. The fish are non-scaly with smooth and moistened skin and have dual modes of respiration i.e. aquatic and aerial. The aquatic respiration is solely performed by highly vascularised four pairs of gills situated in suprabranchial chamber on either side of the head. The moistened skin and air-breathing organs constitute accessory respiratory organs in air-breathing fishes [13], [14] including C. gariepinus. The air-breathing organs also called dendritic organs, are located on 2ndand 4thgill arches in suprabranchial chamber and are intended for aerial respiration. The presence of dendritic organs enables the fish to deal with situations of stress caused due to hypoxia and chemical agents/pollutants in surrounding water. All three organs (gills, dendritic organs and skin) constitute surface organs in fish as these organs are in intimate with the external environment, the water and form good sites for entry of any xenobiotic including the clove oil that mainly enters through the gills and skin [15].
In fish, glycoconjugates synthesis and secretion on surface is one of the unique properties of all the three surface organs (gills, dendritic organs and skin) [2], [4], [5], [6], [7], [9], [14]. These glycoconjugates are synthesized and secreted from surface epithelial cells and more specifically, the mucous or goblet cells. The mucous cells and slime (secretions of epithelial cells and mucous cells) change their chemical properties as per the need and therefore form first line defence system in the fish [16], [17]. The glycoconjugates form chemical complexes with xenobiotic substances and other harmful microorganisms including bacteria and limit their entry into the fish body. The glycoconjugates are also soluble in surrounding aqueous medium and thus help the fish in getting rid of harmful substances and hence are considered good for biomarker studies. In this research paper, an effort has been made to utilize glycoconjugates of surface organs of C. gariepinus as assessing tool for evaluation of effects caused clove oil anaesthesia.
2. Materials and Methods
2.1. Fish Selection, Procurement and Maintenance in Laboratory Conditions
The fresh water catfish, Clarias gariepinus was found available in live condition in fish markets of Ulhasnagar City, District Thane, Maharashtra, India. Live and healthy C. gariepinus (both male and female) were procured, and washed with a concentration of 0.1 ppm of potassium permanganate (KMnO4) to disinfect the fish if any before stocking in laboratory. After washing, the fish were transferred to clean and well dried glass aquaria of twenty litre capacity having plain tap water mixed with 1 ppm antibiotic ampicillin so that the chances of microbial infection, if any can be minimized. The treatment with ampicillin was however restricted to only first day of stocking for acclimation. The fish were regularly fed with minced goat liver and water was renewed every after 24h of expiry during entire period of acclimation.
2.2. Experimental Protocol
After acclimation for fifteen days in laboratory conditions, the fish having total length 30 ± 2 cm and total weight 200 ± 5 g were transferred (total 4 fish for each concentration of clove oil) to glass aquaria having concentrations of 0.07 ml/l, 0.08 ml/l, 0.1 ml/l, 0.12 ml/l, 0.15 ml/l, 0.16 ml/l, 0.2 ml/l, 0.24 ml/l, 0.27 ml/l and 0.3 ml/l of clove oil (LOBA CHEM. PVT. LTD, MUMBAI, Minimum assay 85%) in tap water (temperature 34°C, pH 7.3 and dissolved oxygen 7.64 mg/l). Clove oil was first mixed with equal volume of distilled water in a separate test tube with vigorous shaking up to fusion point and then incorporated into aquarium. Induction and recovery periods and behavioural changes for each concentration of clove oil were recorded (data under process of publication). The clove oil concentration (0.16ml/l) that causes effective anaesthesia in C. gariepinus with minimum mean induction period (3.47minutes) and maximum mean recovery period (1.10 minutes) was selected for present study assuming that the anaesthesia at this concentration of clove oil will be less fatal to fish and will provide sufficient time to investigators for handling operations and surgery. The fish when fully anaesthetized and did not show any response to a sharp pinch in caudal region were sacrificed by cervical dislocation, and the surface organs (gills, dendritic organs and skin,) were isolated, rinsed in normal saline to remove the blood clots present, if any and fixed in Bouin’s fluid [18] and 10% neutral buffered formalin [19] for routine histopathology study and study of glycoconjugates using special stains. The results obtained were compared with that of control fish.
2.3. Tissue Processing
The fixed gills, dendritic organs and skin of both control and experimental (anaesthetized) fish were washed with 70% ethyl alcohol to remove fixatives and other unwanted materials if any, dehydrated following graded ethyl alcohol and stored in Cedar Wood Oil for further processing. The tissues were cut into pieces of appropriate sizes, washed with xylene and transferred to molten paraffin wax (melting point 58 – 60°C) for the purpose of infiltration and finally the embedding was done using paraffin wax. The sections of 6 µm thick were cut using rotary microtome fitted with disposable blade. The paraffin sections were spread on digital hot plate at 40°C for spreading and evaporation of water. The drying of sections was done at room temperature for four days before staining.
2.4. Histopathology Study
Routine histopathology study was done on serial paraffin sections stained with Hematoxylin and Eosin (H/E) following procedure of Ehrlich, 1886 [20].
2.5. Glycoconjugates Study
Some special stains were applied for chemical localization of various glycoconjugates in the gills, dendritic organs and skin. Serial paraffin sections were stained with Periodic Acid Schiff (PAS) [21] for carbohydrate moieties (Glycoproteins with oxidizable vicinal diols and/or glycogen) and Alcian Blue pH 2.5 (AB pH 2.5) [22] and Alcian Blue pH 1.0 (AB pH 1.0) [23] for acidic glycoproteins (Glycoproteins with carboxylic groups and/or O-sulphated esters) and combined Alcian Blue pH 2.5 and Periodic Acid Schiff [22] for differentiation of acidic and neutral glycoproteins (Glycoproteins with mixture of oxidizable vicinal diols and/or glycogen and carboxylic groups and/or O-sulphated esters).
2.6. Photomicrographs
All photomicrographs were captured using Trinocular Research Microscope (Infralab Pvt. Ltd., Chandigarh, India) attached with 10 megapixel Camera. The photomicrographs were analysed with help of image analysis software Biovizard version 4.1.
3. Results
3.1. Behavioural Study
Following exposure to lower concentrations of clove oil (below 0.07 ml/l), fish showed no any change in behaviour. The fish after normal swimming settled on bottom of glass aquarium and showed normal breathing. The anaesthesia was not observed below 0.07 ml/l of clove oil concentration even after placing the fish in aquarium for longer duration (30minutes). The behavioural changes observed at 0.07 ml/l and subsequent higher concentrations of clove oil include erratic swimming, bubbling, rubbing to side and bottom walls of glass aquarium, protrusion of head above water surface, increased opercular movements and partial loss of equilibrium. The erratic swimming and opercular movements however became more intense at higher concentrations (0.1 ml/l, 0.12 ml/l, 0.15 ml/l, 0.16 ml/l, 0.2 ml/l, 0.24 ml/l, 0.27 ml/l and 0.3 ml/l) of clove oil. At later stages of exposures, the fish however became inactive and sank on bottom of aquarium showing decreased opercular movements. The anaesthesia was observed at concentrations of 0.07 ml/l, 0.08 ml/l, 0.1 ml/l, 0.12 ml/l, 0.15 ml/l, 0.16 ml/l, 0.2 ml/l, 0.24 ml/l, 0.27 ml/l and 0.3 ml/l of clove oil however the period of induction and recovery varied for each concentration. The effective anaesthesia in fish was induced when the fish became fully static on bottom of aquarium with ventral surface (belly) up and dorsal surface down (state of complete loss of equilibrium) and ceased opercular movements. The various behavioural changes recorded during exposures causing effective anaesthesia in C. gariepinus include:
(1). Erratic swimming with constant rubbing of body to side walls of glass aquarium, protrusion of head above water surface, increased opercular movements.
(2). Fish settled on bottom and became almost static, increased opercular movements, equilibrium normal.
(3). Decreased opercular movements, slight loss of equilibrium.
(4). Opercular movements ceased, equilibrium lost, dorsal surface up and ventral surface down.
The anaesthetized fish when transferred to another aquarium having plan water for the purpose of recovery, after expiry of some time (recovery period) showed opercular movements followed by swimming in same orientation with ventral surface up and then lateral swimming. After, the fish became normal with dorsal surface up and showed normal opercular movements hence normal breathing.
3.2. Glycoconjugates Study
3.2.1. Skin
The skin of control C. gariepinus is composed of outer epidermis and inner dermis. The epidermis is stratified consisting of single layer of epithelial cells (EC) in the outer most layer. The middle layer of epidermis is entirely composed of large, eosinophilic, protein rich club cells. The oval or flask shaped glycoconjugates synthesizing mucous cells remain intermingled among the epithelial cells of outer most layer and club cells of middle layer (figure1a). The inner most basal layer of the epidermis is composed of many layers of epithelial cells (figure1a). The mucous cells (MCs) were stained almost negatively with
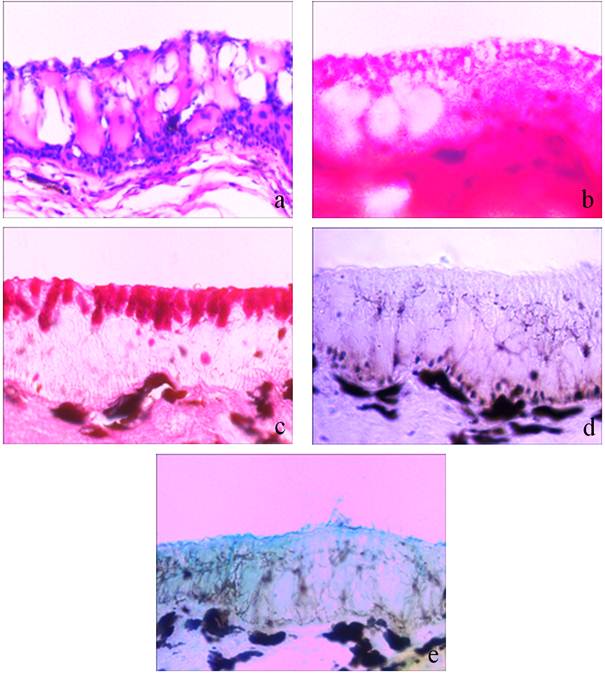
Figure 1. Photomicrographs of vertical section of skin of Clarias gariepinus showing histopathological and histochemical changes in response to anaesthetic effect of clove oil.
Figure 1a. Normal histomorphology with stratified epidermis consisting of outer most layer, middle layer and basal layer. Note distribution of mucous cells among epithelial cells in outermost layer and club cells in the middle layer of epidermis. (Hematoxylin/Eosin)(400X)
Figure 1b. Alerted histomorphology under anaesthetic effect of clove oil. Note decreased density of mucous cells. (Eosin) (400X)
Figure 1c. Normal distribution of mucous cells. (Periodic Acid Schiff) (400X)
Figure 1d. Almost negatively stained mucous and epithelial cells. (Periodic Acid Schiff) (400X)
Figure 1e. Alcian Blue pH 2.5 positive epithelial cells in outer most layer and slime at surface. (Alcian Blue pH 2.5) (400X)
Alcian Blue pH 1.0, weakly to moderately with Alcian Blue pH 2.5, strongly to very strongly with Periodic Acid Schiff (figure 1c) and strongly with combined Alcian Blue pH 2.5 and Periodic Acid Schiff.
Following exposure to clove oil causing effective anaesthesia, there was no significant change in histomorphology of the skin except density and dimensions of the mucous cells which was decreased greatly. However at some places of the epidermis especially in middle layer, large sized mucous cells with full of contents were noticed (figure 1b). The mucous cells and their contents stained negatively with Alcian Blue pH 1.0, Alcian Blue pH 2.5 and Periodic Acid Schiff staining techniques (figure 1d). Alcian Blue pH 2.5 positive slimy layer in the form of small patches was often noticed on surface of the skin (figure 1e).
3.2.2. Gills
In control C. gariepinus, there are four pairs of gills which are located in suprabranchial chamber of either side. Two rows of gill filaments (primary gill lamellae, PL) remain attached on each gill arch. Each primary gill lamella bears a series of alternately arranged secondary lamellae (SL) on its either side. The secondary lamellae are made up of alternately arranged blood channels and supporting pillar cells. A thin layer of epithelium covers the blood channels – pillar cells systems. The epithelial layer also forms a barrier layer between the vascular components (blood channels) and surrounding water. The blood cells flow within the blood channels (figure 2a). The mucous cells are mostly present in the primary lamellae. The mucous cells stained moderately to strongly with Periodic Acid Schiff and weakly to moderately with Alcian Blue pH 2.5 and Alcian Blue pH 1.0.
In fish exposed to clove oil, the epithelial layer covering both primary gill lamellae and secondary lamellae became very thin however the ladder like arrangement of blood channels – pillar cells remained intact. Epithelial lifting and sloughing of epithelial layer (figures 2b and 2c), and oozing of blood at surface of gill was noticed. Some unidentified eosinophilic spots were often seen in epithelial layer of primary gill filament and in between the two adjacent secondary lamellae (figure 2b). The density and dimensions of mucous cells decreased greatly and showed almost negative staining reaction with Alcian Blue pH 1.0, Alcian Blue pH 2.5 and Periodic Acid Schiff stains.
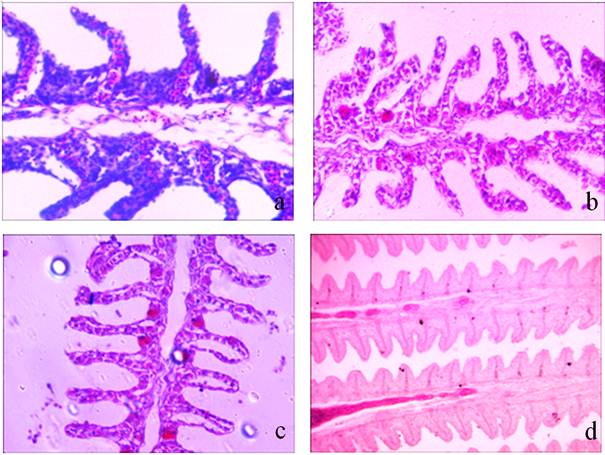
Figure 2. Photomicrographs of transverse section of the gills of Clarias gariepinus showing histopathological and histochemical changes in response to clove oil anaesthesia.
Figure 2a. Normal histomorphology with ladder like arrangement of blood channels and supporting pillar cells. (Hematoxylin / Eosin) (400X)
Figure 2b. Alerted histomorphology due to anaesthesia of clove oil. Note wear and tear of respiratory epithelium and sloughing of epithelial cells at the surface. (Hematoxylin/Eosin) (400X)
Figure 2c. Note epithelial lifting. Also note unidentified eosinophilic spot/cell body in epithelial layer of primary gill filament. (Hematoxylin/Eosin) (400X)
Figure 2d. Periodic Acid-Schiff (PAS) positive staining of epithelial and mucous cells. Note newly formed mucous cells showing more positive reaction with Periodic Acid Schiff. (100X).
3.2.3. Dendritic Organs
The two pairs of dendritic organs are located, one each on the 2nd and 4th gill arches. The terminals or knobs of each dendritic organ are composed of a vascular region having blood channels supported by alternately arranged pillar cells. The vascular regions remain separated by U-shaped non-vascular regions. The entire vascular and non-vascular regions in dendritic organs are covered externally by a thin epithelial layer forming an inter face between the vasculature and external surrounding water. The dendritic organs being modified gills share most of the structural components except a core of cartilage which supports the dendritic organs from inner side. The mucous cells remain distributed in the epithelial cells of non-vascular regions (figure 3a). The mucous cells stained moderately to strongly with Periodic Acid Schiff, weakly to moderately with Alcian Blue pH 1.0 and Alcian Blue pH 2.5 and moderately to strongly with combined Alcian Blue pH 2.5 and Periodic Acid Schiff giving violet colouration (figure 3c).
The clove oil anaesthesia caused flow of more blood through blood channels leading to congestion, haemorrhage and oozing of blood on the surface (figure 3b). The mucous cells density remained almost same. The large sized mucous cells (mucous cells hypertrophy) significant change in density and area occupancy. The staining property of mucous cells was altered showing more affinity for acidic glycoconjugates. The mucous cells and their contents stained strongly to very strongly with Alcian Blue pH 1.0 and Alcian Blue pH 2.5 and combined Alcian Blue pH 2.5 and Periodic Acid Schiff giving bluish violet colouration (figures 3d and 3e).
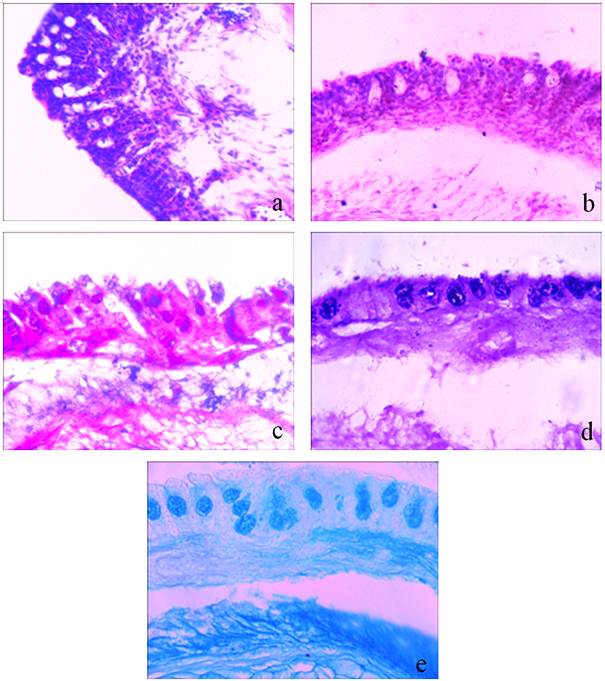
Figure 3. Photomicrographs of transverse section of air-breathing organs of Clarias gariepinus showing histopathological and histochemical changes in response to anaesthetic effect of clove oil.
Figure 3a. Normal histomorphology with ladder like arrangement of blood channels and supporting pillar cells. (Hematoxylin/Eosin) (400X)
Figure 3b. Alerted histomorphology under anaesthetic effect of clove oil. Note congestion of blood channels with blood cells and oozing of blood at surface. (Hematoxylin/Eosin) (400X)
Figure 3c. Normal distribution of mucous cells. (Alcian Blue pH 2.5/Periodic Acid Schiff) (400X)
Figure 3d. Darkly stained mucous cells showing more affinity towards Alcian Blue pH 2.5 staining. Note precipitation of content of mucous cells. (Alcian Blue pH 2.5/Periodic Acid Schiff) (400X)
Figure 3e. Alcian Blue 2.5 positive mucous cells. (Alcian Blue pH 2.5) (400 X)
4. Discussion
The most important toxicity effects following exposure to clove oil that causes effective anaesthesia (state of anaesthesia sufficient for handling and surgical operations) in Clarias gariepinus is the suppression of mucogenic activity resulting in decrease in density and dimensions of mucous cells and secretion of slime on surface of all the three surface organs, the gills, dendritic organs and skin (figures 2d, 3d, 3e, 1d and 1e). The suppression of mucogenic activity is however more significant in the gills and skin. This may perhaps be due to more delicate, highly vascularised and lipoid nature of these two vital organs. The mucous cells and the slime on surface stains positively with Alcian Blue pH 2.5 showing more affinity for glycoconjugates incorporated with carboxylic groups and sulphated esters (figures 1e, 3d and 3e). The clove oil present in anaesthetic bath (the aquarium) perhaps increases the solubility of mucus and other epithelial secretions into the surrounding water and thus prevents their (mucus and epithelial secretions) deposition onto the surface so that more clove oil can easily penetrate, distribute throughout the body and produce effective anaesthesia. The other toxicopathological manifestations caused due to clove oil anaesthesia include wear and tear and sloughing of outer surface lining (epithelium) of gills (figure 2b), lifting of gill epithelium (figure 2c) and oozing of blood from damaged blood channels in dendritic organs (figure 3b). The rapid penetration of clove oil across the gill filaments (both primary and secondary gill filaments) may be one of the reasons for damage and lifting of the gill epithelium. The simultaneous increase of blood flow within the blood channels of dendritic organs enhances oxygen uptake from air and compensate oxygen deficiency caused due to disruption of gill epithelium and ceased opercular movements.
Cove oil produces its anaesthetic effect after absorption through the surface organs mainly through the gills and skin of fish (in non scaly fish as in Clarias gariepinus, the present fish model) [15], [24], [25], [26]. In fish, skin and more specifically the gills are the most delicate and vascularised organs having a separation of few micrometers between the blood and external water [27]. The gills are also the important organs for exchange of gases between external and internal milieu, excretion of harmful nitrogenous substances generated into the body after several metabolic reactions and osmoregulation. Clove oil being lipophilic in nature easily penetrates the gill epithelium and skin, enters the blood stream and distributes throughout the body however the final distribution of clove oil depends on its tissues/cells affinity.
The information on toxicity of clove oil on vital organs and particularly the surface organs of the fish due to effects of anaesthesia is meager. However the toxicity studies of clove oil on mammals have revealed that the acute oral, dermal and inhalation toxicity is low. Acute toxic effects at high doses of clove oil include destruction of the gastric mucosa [28], capillary haemorrhaging in dogs [29], gastric inflammation and depression of secretory capacity [30], liver discoloration and mottling in rats [31] and liver congestion in dogs [32].
The decrease in mucous cells density and dimensions is more significant in the skin and gills. This (decreased mucous cells density and dimensions) results in less production of mucus and hence slime on surface of skin and gills. This over all may facilitate the absorption of more and more clove oil through these surface organs (skin and gills) in order to produce effective anaesthesia. Several toxicological studies have confirmed that the slime and more specifically the glycoconjugates present in it have ability to form complexes of varying degree with external chemical substances and xenobiotics and prevent their absorption into the body [4], [5], [6], [7], [9], [14]. The acids and sulphates groups of glycoconjugates may react with hydroxyl group of eugenol, the main ingredient of clove oil and form complex compounds in order to minimize clove oil entry through these surface organs.
The mechanism of clove oil anaesthesia in fish is still unexplored. The unexpected behaviour shown by C. gariepinus such as erratic swimming, bubbling, rubbing to side and bottom walls of aquarium, protrusion of fish head above water surface following exposure to clove oil indicates that clove oil, at least at initial stages of anaesthesia, is irritating and stress producing in nature. Clove oil impairs the normal functioning of gills by damaging gill epithelium (respiratory barrier) leading to decreased respiratory efficacy. To compensate these changes, the blood flow in dendritic organs increases (figure 3b) and the fish often come at surface to extract aerial oxygen.
The clove oil causes effective anaesthesia in air-breathing fresh water catfish C. gariepinus at different concentrations of exposures. The anaesthesia is however time related and may lead to death when fish are kept for longer time. The surface organs (skin, gills and dendritic organs) which form the main routes for entry of clove oil in water are perhaps the main sufferer organs in C. gariepinus during the exposure. The density and dimensions of mucous cells decreases significantly in gills and skin. The glycoconjugates synthesized and secreted on surface show more affinity for carboxylic group and sulphated esters.
Acknowledgement
Financial support to one of the authors Dr. Ajai Kumar Singh from the University of Mumbai, Mumbai, India under University Research Grant Scheme is fully acknowledged. The authors are also indebted to the Principal of the College and Head of Zoology Department for providing necessary infrastructure facilities required to complete this project.
References
- Chandra S and Banerjee TK. 2003. Histopathological analysis of the respiratory organs of the air-breathing catfish Clarias batrachus (Linn.) exposed to the air. Acta ZoologicaTaiwanica14: 45-64.
- Andrade VM, Silva JD, Silva FR, Heuser VD, Dias JF, Yoneama ML, Freitas TR 2004. Fish as bioindicators to assess the effects of pollution in two southern Brazilian rivers using the Comet assay and micronucleus test. Environ Mol. Mutagen. 44 (5): 459-68.
- Birungi Z, Masola B, Zaranyika MF, Naigaga I, Marshall B 2007. Active biomonitoring of trace heavy metals using fish (Oreochromis niloticus) as bioindicator species. The case of Nakivubo Wetland along Lake Victoria. Physics and Chemistry of the Earth, Parts A/B/C 32: 1350-1358.
- Singh AK and Banerjee TK. 2008a. Toxic impact of sodium arsenate (Na2HAsO4.7H2O) on skin epidermis of the air-breathing catfish Clarias batrachus (Linn.)" Veterinarski Arhiv 78: 73-88.
- Singh AK and Banerjee TK 2008b. Recovery of damages in the skin of arsenic exposed Clarias batrachus (Linn.) following withdrawal of the stress. Iran J. Sci. Health Eng. 5: 17-224.
- Singh AK and Banerjee TK 2008c. Arsenic induced biochemical alterations in the respiratory organs (gills, ABOs and skin) of the walking catfish Clarias batrachus Linnaeus. Biological Research 41: 341-350.
- Singh AK and Banerjee TK 2009. A study on carbohydrate moieties of the gills and air-breathing organs of the walking catfish Clarias batrachus (Linn.) following exposure to arsenic. Toxicological and Environmental Chemistry. 91: 43-52.
- Naigaga I, Kaiser H, Muller WJ, Mumhumuza E 2011. Fish as bioindicators in aquatic environmental pollution assessment: A case study in Lake Victoria Wetlands, Uganda. Physics and Chemistry of Earth Parts A/B/C 36 (14): 918-928.
- Singh AK and Banerjee TK 2014. Histopathological and histochemical study on gills of the fresh water walking catfish Clarias batrachus (Linn.) following exposure and withdrawal of arsenic stress.Int. J. Intsci. Inn. Tech., 3 (4): 12-19.
- Authman MMN, Zaki MS, Khallaf EA, Abbas HH 2015. Use of Fish as Bio-indicator of the Effects of Heavy Metals Pollution. J Aquac. Res. Development 6: 328.
- Schmid R 1972. A resolution of the Eugenia-Syzygium controversy (Myrtaceae). Amer J. Bot. 59 (4): 423–436.
- Shen Q, Li W, Li W 2007. The effect of clove oil on the transdermal delivery of ibuprofen in the rabbit by in vitro and in vivo methods. Drug Dev. Ind. Pharm. 33 (12):1369-74.
- Munshi JSD 1961. The accessory respiratory organs of Clarias batrachus (Linn.). J. Morphology. 109 (2): 115-139.
- Banerjee TK 2007.Histopathology of respiratory organs of certain air-breathing fishes of India. Fish Physiol. Biochem., 33: 441.
- Javahery S, Nekoubin H, Moradlu AH 2012.Effect of anaesthesia with clove oil in fish (review). Fish Physiol. Biochem. 38: 1545–1552.
- Esteban MA 2012. An over view of the immunological defences in fish skin I S R N Immunology. Pages 29.
- Gomej D, Sunyer JO, Salinas I 2013. The mucosal immune system of fish: the evolution of tolerating commensals while fighting pathogens. Fish Shell fish Immunol.35 (6): 1729-1739.
- Bouin P 1897. Phenomenes Cytologique Anoramoux Dans. I Histogenes. Nancy.
- Lillie RD and Fullmer HM 1976. Histopathologic technic and Practical Histochemistry, 4th edition, New York: McGraw-Hill.
- Ehrlich P 1886. Fragekasten. Z. Wiss. Mikrosk. 3: 150.
- McManus JFA 1948. Histological and Histochemical uses of periodic acid. Stain Technol., 23: 99-108.
- Mowry RW 1956. Alcian blue technique for histochemical study acidic carbohydrates. J. Histochem. Cytochem. 4: 407-408.
- Lev R and Spicer SS 1964. Specific staining of sulphate groups with Alcian blue at low pH. J. Histochem. Cytochem., 12:309.
- McFarland WN 1959. A study of the effects of anesthetics on the behavior and physiology of fishes. Publication of the Institute of Marine Science, University of Texas at Austin 6:23–55.
- Hunn JB, Allen JL 1974. Movement of drugs across the gills of fishes. Ann. Rev. Pharmacol. 14: 47–55.
- Ferreira JT, Schoonbee HG, Smit GL 1984. The uptake of the anaesthetic benzocaine hydrochloride by the gills and skin of three fresh water fish species. J. Fish Biol. 25: 35–41.
- Wood CM and Soivio A 1991. Environmental effects on gills functions: An Introduction. Physiological Zoology. 64: 1-3.
- Hitchcock, CR 1952. Failure of eugenol and heat to potentiate gastric tumor induction by 20-methylcholanthrene in mice, J. Natl.CancerInst., 12 (4): 723-733.
- Hartiala KJW, Pulkkinen M, Ball P 1966. Inhibition of ß-D Glucosiduronic acid conjugation (rat and guinea pig) by eugenol, Nature, 210 (5037), 739-740.
- Sober HA, Hollander F, Sober EK. 1950. Toxicity of eugenol; Determination of LD50 on rats, Proc. Soc. Exp. Biol. Med., 73: 148-151.
- Taylor JM, Jenner PM, Jones WI 1964. A comparison of the toxicity of some allyl, propenyl and propyl compounds in the rat, Toxicol. Appl. Pharmacol., 6: 378-387.
- Lauber FU, Hollander F 1950. Toxicity of the Mucigogue, eugenol, administered by stomach tube to dogs, Gastroenterol. 15 (3): 481-486.

 (A. K. Singh)
(A. K. Singh) 




