| 1. | ||
| 2. | ||
| 3. | ||
| 3.1. | ||
| 3.2. | ||
| 3.3. | ||
| 3.4. | ||
| 4. | ||
Rapid Powder Al and Si Codeposition Assisted by Direct Current Field for AISI 1045 Steel
Xiang Du1, 2, Fei Xie1, 2, Xianbo Pan1, Jing Hu1, 2, *
1Jiangsu Key Laboratory of Materials Surface Science and Technology, Changzhou University, Changzhou, China
2Jiangsu Collaborative Innovation Center of Photovolatic Science and Engineering, Changzhou University, Changzhou, China
Email address
 (Jing Hu)
(Jing Hu) Citation
Xiang Du, Fei Xie, Xianbo Pan, Jing Hu. Rapid Powder Al and Si Codeposition Assisted by Direct Current Field for AISI 1045 Steel. Journal of Materials Sciences and Applications. Vol. 2, No. 5, 2016, pp. 35-38.
Abstract
A rapid powder Al and Si codeposition process was developed forAISI 1045 steel on the basis of conventional pack cementation processesby applying direct current field (DCF) between the treated samples embedded in the powder agents. Optical microscopy (OM), X-ray diffraction (XRD) andoxidation test at 800°C were used to characterize the microstructures, phase constituentsand high temperature oxidation resistance. The results show that DCF cansignificantly enhance powder Al and Si codeposition, lower treating temperature and duration can be used comparing with the conventional pack cementation processes to get the same layer thickness. The intermetallic coatings mainly consisted of Al0.7Fe3Si0.3 and AlFe3 can provide effective protection against high temperature oxidation at 800°C.
Keywords
Codeposition, Pack Cementation, Coatings, Direct Current Field
1. Introduction
AISI 1045 steel has been widely used in many aspects due to its good combined properties. In order to further enlarge its application, surface modification is necessary to improve its properties, especially the anti-oxidation and anti-corrosion properties.
Powder aluminizing and siliconizing cementation belong to powder chemical thermal-diffusion technology, which has long history in the application of anti-oxidation and anti-corrosion. Pack aluminizing and pack siliconizing cementation could improve the oxidation and corrosion resistance of metal materials efficiently [1-4]. With the improvement of science &technology and the development of manufacturing, there are increasing requirements for surface properties of materials. In some cases, single powder aluminizing or powder siliconizing can't meet theadvanced requirements, therefore, the process for Al and Si to simultaneously deposithas been widely explored [5-9].
Unfortunately, the pack-cementation was normally performed at temperatures above 900°C, limited by the diffusion and reaction kinetics involved. Such high temperature treatment inevitably limits the application of pack-cementation [10-14]. Therefore, reducing pack-cementation temperature is of significant value and required for widespread application of the pack- cementation. Our previous research found that direct current field (DCF) has significant effect on energy saving and efficiency improving in powder-pack boriding and powder-pack aluminizing [15-16].
In this work, DCF was employed in powder Al and Si codeposition forAISI 1045 steel, the goal is to clarify whether DCF can enhance powder pack Al and Si codeposition process, and whether the rapidly obtained coating can provide good anti-oxidation property for AISI 1045 steel at high temperature.
2. Experimental
The material used in this work was AISI 1045 steel with the following chemical compositions (wt. %): C: 0.45, Si: 0.17, Mn: 0.52, S: 0.031, P: 0.032 and Fe balance. The specimens were cut and sized to approximately Φ10mm×5mm and then degreased and polished progressively by hand finishing to obtain the same surface condition, the specimens were then ultrasonically cleaned in an ethanol bath for 5 minutes, dried prior to pack cementation process. The pack powders(wt.%) were comprised of 30% FeAl as Al source,20% FeSi as Si source,48% quartzite as inert filler to prevent pack from sintering and 2% NH4Cl as halide activator. The pack powders were dried in the oven at 373 k for 1 h before packing.
Schematic diagram of the main part of the experimental apparatus was shown in Fig. 1. In theprocess of powder Al and Si codeposition assisted by DCF, two electrodes with a distance of 10 mm were buried in pack powders charged into a pot, which was then sealed with a lid by fire clay mortar to prevent access gas from expelling when it was heated to high temperatures. The pot was cured in a furnace for about 1 h at approximately 373 k in order to take out the remaining moisture. The pot was then placed in an electric furnace and applying direct current field at 1023k for 4 h. After 4h heating, the pot was left in the furnace to cool down to room temperature at its natural rate by switching off the power supply. For comparison, a reference sample 12) which was not connected to the circuit was also placed in the container as shown in Fig. 1.
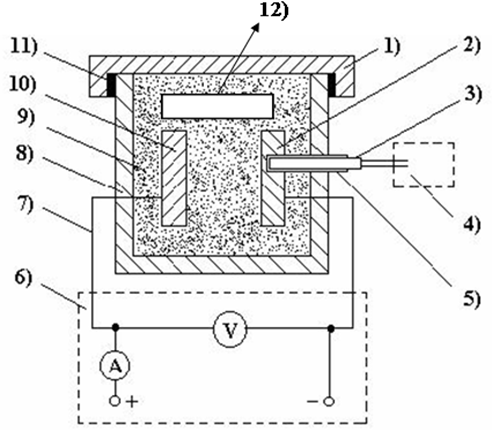
Fig. 1. Schematic of the main part of the experimental apparatus.
1) Lid, 2) Specimens as cathode, 3) Thermo-couple, 4) Device for temperature measuring, 5) Ceramic protection tube, 6) Voltage-controllable DC supplier, 7) Conducting line, 8) Container, 9) Mixture powder agents, 10) Specimens as anode, 11) Sealing Materials, 12) Reference sample
CK-40M OLYMPUS optical microscope was employed for observing the cross-sectional microstructure and investigating the coatings thickness. The phases of the coated layers were identified by Siemens D/max2500PC X-ray diffraction. Cyclic oxidation tests were carried out in air at 800°C,the total oxidation time was 120 h, the sampleweights were measured to 0.0001 g every 10 h until the end of the tests.
3. Results and Discussion
3.1. Cross-Sectional Microstructure and Coatings Thickness
The Cross-sectional microstructures ofAISI 1045 steel after powder Al and Si codeposition at different conditions are shown in Fig.2. It can be seen that the coatings appear to be homogeneous, free of porosity and have good adherence to the substrate. And by comparing (a) with (b),and (c) with (d), it clearly shows that the coating thickness increased almost twice assisted by DCF when the furnace temperature, agents contents and holding time were the same, which means that DCFcan significantly lower treating temperature and duration compared with the conventional pack cementation processes.
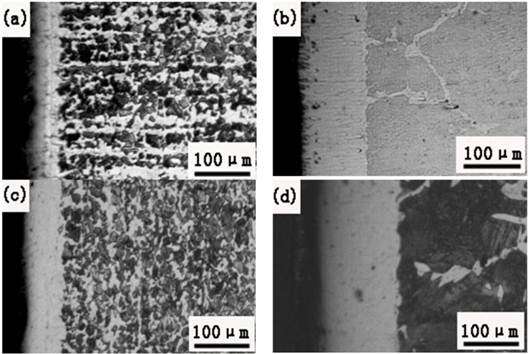
Fig. 2. The cross-sectional microstructures of AISI 1045 steel after powder Al and Si codepositing for 4h at different temperatures with and without DCF (a)700°C, without DCF(b)700°C, with 4A DCF(c)750°C, without DCF(d)750°C, with 4A DCF.
3.2. XRD Analysis
Fig. 3 presents XRD pattern of the AISI 1045 steel specimens treated at 750°C for 4h with and without DCF assisting. It shows that specimens after powder Al and Si codeposition, the main phases of the coatings changed from intermetallics of FeAl and FeSi to Fe3Al and Al0.7Fe3Si0.3 by applying DCF, which indicates that DCF preferably promote active Al atoms to produce and diffuse due to the stronger electric charging property than that of active Si atoms. Therefore, DCF brings out the preferential formation of Fe-Al intermetallics.
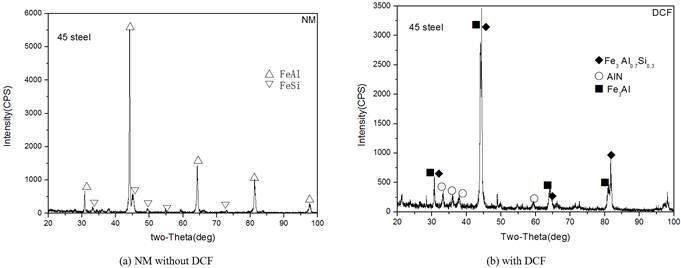
Fig. 3. XRD pattern of the specimens treated at 750°C for 4h by NM and DCF(4A).
3.3. High-Temperature Oxidation Resistance Test
Cyclic oxidation test was carried out in air at 800°C with total duration of 120 h, the sample weights were measured every 10 h. Fig.4 shows the oxidation kinetic curves of the AISI 1045 steel samples powder Al and Si codeposition treated and as-received. It illustrates that the coatings assisted by DCF greatly improved the high temperature oxidation resistance of AISI 1045 steel. The reason is that a compact intermetallic layer comprised of FeAl3 and Al0.7Fe3Si0.3 is formed on AISI 1045 steel surface after powder Al and Si codeposition, which can effectively protect the substrate against oxidation.
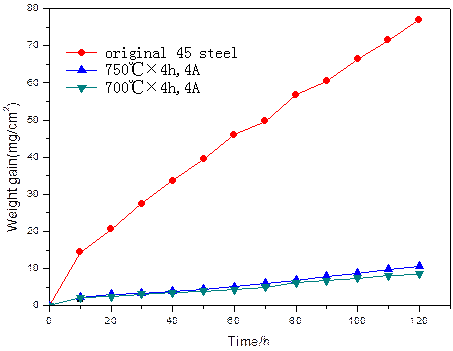
Fig. 4. Oxidation kinetic curves of specimens treated and as-received at 800°C.
3.4. Discussions
The coating thickness increases almost twice assisted by DCF when the furnace temperature, agents contents and holding time are the same as shown in Fig.1, which means that DCF has significant effect on accelerating the diffusion of active atoms, saving energy and improving the usage of powder agents.
Firstly, DCF has thermal effect on agents, cathode and anode specimens which can promote the decomposition of FeAl and FeSi agents, thus increase the concentration of the active Al and Si atoms in the agents. More importantly, the active Al and Si atoms produced are positively charged by DCFand forced to diffuse directionally toward the cathode specimen. This directional diffusion is obviously faster than that in random, and it can also reduce the absorption of the active Al and Si atoms by the inner wall of crucible and the non-working surface of component. Therefore, the concentrations of the active atoms around the surface of the specimen (cathode) can be much higher than that at any other positions, and thus the usage efficiency of the active atoms in the agents is significantly improved, hence promoting the formation of coating layer and resulting in thicker depth.
4. Conclusions
1) DCF can significantly lower treating temperature and duration compared with the conventional pack cementation processes.
2) The main phases of the coatings are Al0.7Fe3Si0.3 and AlFe3 treated by powder Al and Si codeposition with DCF enhancement.
3) The powder Al and Si codeposition coatings assisted by DCF have very good oxidation resistance at 800°C.
Acknowledgements
The work was supported by the National Natural Science Foundation of China under the Grant No. 51271039 and PAPD of Jiangsu Higher Education Institutions.
References