Effects Association of Azadirachta indica Treatment and Age at First Pasture in Goats
Akourki Adamou*, Chaïbou Issa, Sidikou Idrissa Djibo,
Adakal Hassane
Department of Animal Sciences and Techniques, Faculty of Agriculture and Environmental Sciences, University of Dan Dicko Dankoulodo, Maradi, Niger
Email address

(A. Adamou)
*Corresponding author
Citation
Akourki Adamou, Chaïbou Issa, Sidikou Idrissa Djibo, Adakal Hassane. Effects Association of Azadirachta indica Treatment and Age at First Pasture in Goats. American Journal of Agricultural Science. Vol. 3, No. 2, 2016, pp. 21-26.
Abstract
The goal of the present study was to investigate the effects of the age at the first pasture and a treatment based on administration of Azadirachta indica extract in goats. The study was carried out with a total of 50 young red-goats of Maradi. For the grazing experiment, animals were used belong to three groups (G) defining their age at the first pasture; G1: two months; G2: 3 months and G3: four months. For the treatment with Azadirachta indica, five lots were formed, L1, L2, L3 receiving 250 mg/kg body weight respectively every 24, 48 and 72 h, W1 lot received 2.5 ml water/ kg every 72 h, and W2 was subjected to contention and oral fluid administration. The weight gain was followed each week, coccidia and strongyles infestation levels were determined by fecal egg count and the hematocrit was assessed. Results showed: for the treatment effects, no significant differences have been found nor in weight gain (p = 0,340), hematocrit volume (p = 0,210), coccidia infestation (p = 0,153), neither in strongyles infestation (p = 0,704). About age at the first pasture effects, it was found in animals of G1 the best weekly weight gain (385.5 ± 298.2 g/day (p < 0.05), a low intensity of coccidia infestation with 5832.43 oocyte/g of feces (p < 0.05), and a high strongyles infestation with 3859.45 ± 2862.76 eggs/g of feces. To expect better performance, young goats have to graze as earlier as possible, Azadirachta indica dosage and treatment frequencies are to be improved.
Keywords
Coccidiosis, Strongylosis, Azadirachta indica, Pasture, Mardi Red Goat
1. Introduction
In tropical countries, animal feeding, herds’ management and disease control are within the most often confronted problems. Goat breeding has many strengths and potentials that are underutilized when considering the needs [1]. Among various pathologies, parasitism causes enormous economic loses in livestock production. It have been mentioned that the level of parasitic infestation of grazing goats is such that it could result in loss of production from 15 to over 50% morbidity and mortality [2]. Due to the low immunity development, a high frequency of treatments is commonly adopted with chemical products as benzimidazoles or levamisoles for helminthes, and Decox for coccidiosis. This intensive use of drugs is origin of emergence of drug resistance, environmental contamination and reduction of pasture areas [3].
However, for parasites control, Azadirachta indica have been indicated elsewhere in veterinary medicine [4]. Commonly called Neem, A. indica has been subject of a number of studies for the treatment or medical control in many research areas such as mice udder cancer [5], malaria [6], filariasis [7], etc. Many other studies showed the antimicrobial effects on ectoparasites [8], endoparasites [9,10], and particularly in the fight against the intestinal parasites Eimeria [11,12].
Coccidiosis is common in most animal species. In avian, some anti-coccidia vaccines have been developed and sold commercially [13]. In goats it is one of the most important diseases in terms of economic losses. It have been demonstrated that coccidia pathogenicity is higher in young [14], that it destroys the antioxidant system [15] and attacks the jejunum portion of the intestine causing diarrhea, dehydration and body weight loss [16]. Otherwise, it have been noted a possible toxic effect of Neem extract generating organ lesions [17].
The understanding of goat’s response by combination of administration of extract of A. indica and first grazing period management could provide useful information for sustainable livestock development. Thus, the present work is conducted to study the growth and health behavior of red-goats depending on the age at the first pasture, and on the treatment with extracts of A. indica.
2. Materials and Methods
2.1. Animals
The experimentation was conducted in the Secondary Center of Goat Multiplication of Maradi (Niger). This study involved 50 young red-goats with an average age of 3.90 months. Food supplementation consists of wheat bran, Piliostigma reticulatum pods and alfalfa hay.
Young animals used belong to three groups (G) defining their age at the first pasture: G1: two months (n = 21); G2: 3 months (n = 15) and G3: four months (n = 14).
2.2. Neem Extracts Preparation and Oral Administration
For extract preparation, 25 g of fresh Azadirachta indica (A. indica) leaves and 25 g of fresh seeds are crushed, pounded and diluted in 500 ml of water. The solution is then conserved at 4°C overnight before the treatment. Per-os administration root was adopted.
For the oral treatment, the same goats were divided into five lots (L) of 10 receiving 250 mg/kg each, every 24 h for L1, every 48 hours for L2 and every 72 hours for L3. W1 (witness lot 1) received only 2.5 ml of running water /kg every 72 h, and W2 (witness lot 2) was without any constraint and fluid administration.
Goats were regularly weighed to determine the average weight gain. The various weighing and analysis are realized during the following periods:
• DE1: the first day of the experiment;
• CD: the ending or cessation day of oral administration;
• WCD1: one week after cessation day of oral administration;
• MCD1: one month after cessation day of oral administration;
• MCD2: two months after cessation day of oral administration.
2.3. Blood Sampling and Hematocrit Determination
Blood was sampled each weighting day, and collected from the caudal vein in heparinized tubes, conserved at 4°C before sending to the laboratory for centrifugation and analyses. The assay was performed according to the method described in 1993 [18].
2.4. Feces Collection and Parasitism Determination
Fresh feces, blood samples were collected and animals weighting were done the same day. Feces was conserved in a hermetic plastic tube and conserved at 4°C prior to the laboratory for coccidia oocystes and strongyle eggs count. The assay was performed according to the method described by McMaster, quantitative method based on the flotation principle.
2.5. Statistical Analyses
Data were analyzed using Statistical Package for Social Scientists for Windows, 20 [19] and an analysis of variance (ANOVA) was carried out. Duncan test was used to compare arithmetic means. P values < 0.05 and < 0.01 were considered statistically significant.
3. Results and Discussion
3.1. Treatment Effects of Azadirachta Indica
Table 1. P value of multivariate linear analysis.
| Effects | WAG | Hematocrit | Strongyles | Coccidia |
| Lots of treatment | 0,340 | 0,210 | 0,704 | 0,153 |
| Age of group | 0,000 | 0,864 | 0,001 | 0,000 |
| Period of study | 0,017 | 0,004 | 0,132 | 0,000 |
| Lots of treatment*Age of group | 0,149 | 0,004 | 0,401 | 0,006 |
| Lots of treatment*Period of study | 0,787 | 0,518 | 1 | 0,040 |
Age of group*Period of
study | 0,005 | 0,000 | 0,155 | 0,000 |
WAG: Weekly average of weight gain
According to the P-value (Table 1), no significant differences were found in weekly weight gain between groups (p = 0.340). Nevertheless, the weight gain seems lower in groups medicated each of 48 h (L2: 192.2 ± 159.6g/week) and 72 h (L3: 189.8 ± 176.5g/week) compared to medicated lot each 24 h (L1: 289 ± 159.6g/week). The volume of hematocrit did not change statistically between groups (p = 0.210). The same finding is made for both coccidia oocystes (p = 0.153) and strongyle egg count (p = 0.704).
However, [12] reported that, a 4 days consecutive administration of a dose of 500 mg/kg of Neem extracts in mice had an anti-coccidial effect and also allowed weight maintenance of artificially infected coccidiosis. Also, in the study conducted in chickens, it was observed after 9 consecutive treatment days, a significant improvement of performances after Eimeria tenella infection and a reduction of clinical signs of coccidiosis in birds treated with 100 mg/kg of Neem leaves extracts [11].
In the present study, the lack of significant clinical change or difference between treated and not treated goats could be likely due to the frequency of treatment, or an insufficient dose of A. indica. Or, a probable difference could be found in coccidia species present in these goats and those present in chickens and mice, suggesting differences in sensitivity to the Neem active substance. Differences in results could also be due to technics or methods used in each study for the preparation of Neem extract. Deep differences are noted with the method of methanol extraction of chemical constituents [12].
Stress is a factor that could influence high infestation. It have been registered that the stress due to the treatment administration (group W1) did not have negative impact on goats performance when compared with group W2 that did not undergone stress. This means that the consecutive stress did not induce high parasitism that can negatively affect growth.
3.2. Effect of Age at First Pasture Period
Age at first pasture influenced significantly all parameters studied (p ≤ 0.001) except hematocrit volume (Table 1) that remained constant between the three groups (p = 0.864).
Thus, at ages 1 and 3, goats had the highest and lowest average weekly weight gain, respectively 385.5 g and (–) 54.2 g. This difference observed between the two groups is maintained within the different treatment lot (Figure 1). The general tendency is a decrease of the weight gain when increasing the age at the first pasture. This could be concomitantly due to food ingestion related to pasture associated with milk consumption of the young goats.
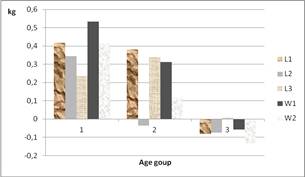
Figure 1. Variation in the weekly weight gain in treated groups according to the age at first pasture.
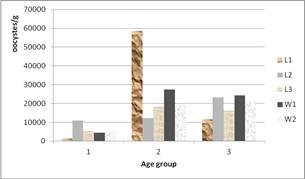
Figure 2. Infestation intensity due to coccidia in treated groups according to the age at first pasture.
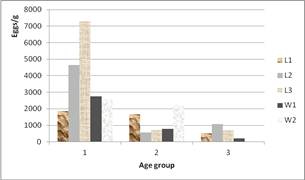
Figure 3. Infestation intensity due to strongyles in treated groups according to the age at first pasture.
In addition, the low weight gain obtained in age group 3 may be associated with a lack in hygiene facilities. Indeed, inadequate frequency in the litter removing or changing combined with an insufficient disinfection are observed. That facilitates the development of microorganisms such as coccidia or other pathogenic viruses or bacteria as reported in the center. Elsewhere, no important pathology was noted during the present study. Nevertheless, in these conditions it have been noted higher mortality; 42.85% and 20% respectively in age groups 3 and 2, groups that have highest waiting period in the center before beginning pasture against 0% for age group 1. To the ethological point of view, housing and a prolonged stalling can have negative consequences on goats’ performances such as weight gain reduction [20]. This implies that, so earlier animals begun going in pasture, so sooner will be the improvement of productions.
For coccidia oocystes numbered in feces, it was higher for goats of groups 2 and 3 (p = 0.000), while the minimum was found in group 1. The eggs count per gram for strongyles parasites was highly important (p = 0.001) in goats of age 1. This difference observed within the three groups of age remained in the 5 lots of treatments (Figures 2 and 3). The tendency in figure 2 is an increase of coccidia oocystes when increasing the age before first pasture. And figure 3 shows a tendency of general increasing of strongyle eggs when decreasing age before the first pasture.
The prevalence estimation yielded 97%, 100% and 96% of fecal samples containing coccidia oocystes, and 95.83%, 77.96% and 48% of fecal samples containing strongyles eggs respectively for age groups 1, 2 and 3.
These results of prevalence and intensity of parasitism corroborate with previous studies [14,21,22,23]. When analyzing the infestation due to strongyles, we noted that goats in the age group 1 had more fecal eggs than groups 2 and 3. It is a fact that group 1 was first to go in pasture within an ‘intense’ raining period (July-August) which corresponds to the period presenting a high prevalence and intensity of infestation [24].
Regarding to immunity development in small ruminants, repeated strongyle infestation, or an experimental vaccine induced only low or moderated immunity [25,26]. The degree of infestation is determined about the value of eggs/g. It have been reported values around 500 eggs/g for moderate infestation, within 500 and 1000 eggs/g for high infestation, and very high infestation when it is over 1000 eggs/g [27]. It is a fact that the result of the present study gave values around 3.800 eggs/g for the maximum and around 500 eggs/g for the minimum, showing a high strongyle pressure in pasture. One could suggest the hypothesis that red goats of Maradi have high environmental tolerance and/or develop immunity, so that morbidity or/and mortality due to the parasite are reduced.
As regard to parasites type, it is found that coccidia infestation was more important than the strongyles one. In the same kind of study, it was pointed out in Danish goats a prevalence of 99.6% for coccidia and 69% strongyles [28]. Besides other possible reasons, this difference may be related to the contact duration between animals and their infesting environment which is conductive to development for the one or the other parasite (pasture, stables). Indeed, goats spent an average of 6 hours in pasture that is an adequate exposure conditions, for lungworm larvae ingestion and infestation of goats. For the rest of the day (18 h), the goats are in stables with poor hygiene conditions, that’s especially ideal conditions for the development of the parasite coccidia.
3.3. Monitoring Parasitism Throughout the Study
It’s shown in Figure 4 that, for strongyles eggs the increasing was very slight from the first day of experience to the cessation day, but for coccidia oocystes the curve increase drastically from the same period. Beyond, both of them decreased gradually until the end of the study. However, for the percentage of feces containing strongyles eggs or coccidia oocystes (Figure 5), a slight decrease was observed until one week after cessation, then a slight increase begun and stabilized around 2 month after cessation. For the strongyle eggs, after a slight decrease until 1 day after cessation, it increased slightly before a new step of decreasing until the second month after cessation (Figure 5).
Regarding to the weight gain and hematocrit volume, values decreased from the beginning of the experiment till the first day of cessation for the weight gain, and then increased for the rest of the period. For hematocrit the decreasing continued until one week after cessation before it increased until 2 months after (Figure 6).
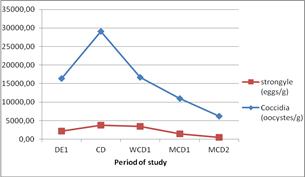
Figure 4. Evolution of the intensity of infestation for each parasite during the study.
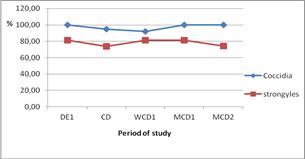
Figure 5. Evolution of the prevalence of the different parasitosis.
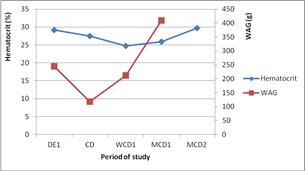
Figure 6. Trends of weight gain and hematocrit.
Towards the end of the experiment, mainly during the second month following the oral administration of extracts, parasitism decreased significantly, probably due to the cessation of rains. That is probably one of the factors that contribute in reducing moisture, development of Strongylidae and multiplication of coccidia. Along that month, a better growth was perceived; remember that at that period, all goats went in pasture.
4. Conclusion
For the main conclusions of the present study, it could be noted that the treatment effects are not as significant as expected, and it seems that administration of greater dose at greater frequency may allow better results. Stress consideration shows no negative impact on goats.
At last, and not less important, a combination of early pasture and A. indica treatment gave a tendency of performance improvement, which may be confirmed by future studies.
Acknowledgments
The authors would like to thank the direction of Centre Secondaire d’élevage Caprin de Maradi and LABOCEL Zinder for their cooperation and facilitating the animals’ access and laboratory analysis.
We are grateful to Dan Dicko Dankoulodo University of Maradi who granted this study.
References
- Ahuya C. O., Okeyo A. M., Mwangi-Njuru, Peacock C., 2005. Developmental challenges and opportunities in the goat industry in Kenya. Small Rum. Res., 60: 197-206.
- Mandonnet N., Bachand M., Mahieu M., Arquet R., Baudron F., Abinne-Molza L., Varo H., Aumont G., 2005. Impact on productivity of peri-parturient rise in fecal egg counts in Creole goats in the humid tropics. Vet. Parasitol., 134: 249-259.
- Sidikou I. D., Remy B., Hornick J. L., Losson B., Duquesnoy N., Yenikoye A., Beckers J. F., 2005. Le pepsinogène et la prochymosine des bovins: connaissances actuelles, applications et perspectives dans la stratégie de lutte contre les verminoses gastrointestinales. Ann. Méd. Vét., 14: 213-228.
- Dossou A. D., 2008. Effet du tourteau de Neem (Azadirachta indica A. juss) sur les coccidioses aviaires. E. I. S. M. V, Dakar, Thèse, 89 pages.
- Othman F, Motalleb G, Lam Tsuey Peng S, Rahmat A, Basri R, Pei Pei C., 2012. Effect of Neem Leaf Extract (Azadirachta indica) on c-Myc Oncogene Expression in 4T1 Breast Cancer Cells of BALB/c Mice. Cell J., 14(1): 53-60.
- Nagendrappa PB, Naik MP., 2013. Payyappallimana U. Ethnobotanical survey of malaria prophylactic remedies in Odisha, India. J Ethnopharmacol., 19; 146(3): 768-72.
- Mukherjee N, Saini P, Mukherjee S, Roy P, Sinha Babu SP., 2014. In vitro antifilarial activity of Azadirachta indica aqueous extract through reactive oxygen species enhancement.Asian Pac J Trop Med., 7(11): 841-8. doi: 0.1016/S1995-7645(14)60147-4.
- Remedio RN, Nunes PH, Anholeto LA, Oliveira PR, Camargo-Mathias MI., 2015.Morphological effects of neem (Azadirachta indica, A. juss) seed oil with known azadirachtin concentrations on the oocytes of semi-engorged Rhipicephalus sanguineus ticks (Acari: Ixodidae). Parasitol Res., 114(2): 431-44.
- Elavarasu S, Abinaya P, Elanchezhiyan S, Thangakumaran, Vennila K, Naziya KB., 2012. Evaluation of anti-plaque microbial activity of Azadirachta indica (neem oil) in vitro: A pilot study. J Pharm Bioallied Sci., 4(Suppl 2): S394-6.
- Jamra N, Das G, Singh P, Haque M. 2015. Anthelmintic efficacy of crude neem (Azadirachta indica) leaf powder against bovine strongylosis. J Parasit Dis. 2015 Dec; 39(4): 786-8. doi: 10.1007/s12639- 014-0423-9. Epub 2014 Feb 2.
- Toulah FH, Ismeel HA, Khan S., 2010. Effect of treatment with Neem (Azadirachta indica) compared with Baycox drug on the caecum of chicken experimentally infected with Eimeria tenella. J Egypt Soc Parasitol., 40(1): 93-106.
- Dkhil MA, Al-Quraishy S, Abdel Moneim AE, Delic D., 2013. Protective effect of Azadirachta indica extract against Eimeria papillata-induced coccidiosis. Parasitol Res., 112(1): 101-6.
- Dalton, J. P and Mulcahy, G., 2001. Parasite vaccines a reality? Veterinary Parasitology, 98: 149–167.
- Balicka-Ramisz A, Ramisz A, Vovk S, Snitynskyj V., 2012. Prevalence of coccidia infection in goats in Western Pomerania (Poland) and West Ukraine region. Ann Parasitol., 58(3): 167-71.
- Rakhshandehroo E, Razavi SM, Nazifi S, Farzaneh M, Mobarraei N., 2013. Dynamics of the enzymatic antioxidants during experimental caprine coccidiosis. Parasitol Res., 112(4): 1437-41.
- Hashemnia M, Khodakaram-Tafti A, Razavi SM, Nazifi S., 2012. Experimental caprine coccidiosis caused by Eimeria arloingi: morphopathologic and electron microscopic studies. Vet Res Commun., 36(1): 47-55.
- Wang C, Cao M, Shi DX, Yin ZQ, Jia RY, Wang KY, Geng Y, Wang Y, Yao XP, Yang ZR, Zhao J., 2013. A 90-day subchronic toxicity study of neem oil, a Azadirachta indica oil, in mice. Hum Exp Toxicol., 32(9): 904-13.
- National committee for clinical laboratory standards, 1993. Procedure for determining packed cell volume by the microhematocrit method - second edition; Approved Standard. NCCLS Document H7- A2, Vol. 13 n° 9. Villanova PA. 19085 États-Unis.
- SPSS 20.0 pour Windouws, 2011. SPSS inc.
- Fraser A. F., 1990. Farm animal behaviour. An introductory textbook on the study of behaviour as applied to horses, cattle, sheep and pigs, 3e édition. Londres, 196p.
- Zhao GH, Lei LH, Shang CC, Gao M, Zhao YQ, Chen CX, Chen DK., 2012. High prevalence of Eimeria infection in dairy goats in Shaanxi province, northwestern China. Trop Anim Health Prod., 44(5): 943-6.
- Akourki Adamou et Chaïbou Issa, 2013. Possibilités d’utilisation de quelques plantes locales; Allium sativum et Sida cordifolia en médecine vétérinaire. Rapport du projet de recherche scientifique. 17 Pages. UMR SEEF. Université Dan Dicko Dankoulodo de Maradi. Niger.
- Khajuria JK, Katoch R, Yadav A, Godara R, Gupta SK, Singh A., 2013. Seasonal prevalence of astrointestinal helminths in sheep and goats of middle agro-climatic zone of Jammu province. J Parasit Dis., 37(1): 21-5.
- Choubisa SL, Jaroli VJ., 2013. Gastrointestinal parasitic infection in diverse species of domestic ruminants inhabiting tribal rural areas of southern Rajasthan, India. J Parasit Dis., 37(2): 271-5.
- Vercruysse J. and Claerebout E., 2003. Assessment of the efficacy of helminth vaccines. J. Parasitol., 2003, 89 (Suppl.): S202- S209.
- De Maere V., Vercauteren I., Gevaert K., Vercruysse J., Claerebout E., 2005. An aspartyl protease inhibitor of Ostertagia ostertagi: Molecular cloning, analysis of stage and tissue specific expression and vaccine trial. Molecular & Biochemical Parasitology, 141: 81–88.
- Caprin. Strongles digestifs et pulmonaires chez les caprins, 2009. Bulletin de l’Alliance Pastorale, N°793: p.8.
- Holm SA, Sörensen CR, Thamsborg SM, Enemark HL., 2014. Gastrointestinal nematodes and anthelmintic resistance in Danish goat herds. Parasite., 21: 37. doi: 10.1051/parasite/2014038. Epub 2014 Jul 31.

 (A. Adamou)
(A. Adamou) 







