In Vitro Propagation of Sugar Beet Cultivar Frida, Through Encapsulated Different Explants
Roba M. Ismail, Wesam M. Raslan, Gihan M. H. Hussein
Gene Transfer Lab, Plant Genetic Transformation Department, Agricultural Genetic Engineering Institute (AGERI), Agricultural Research Center (ARC), Giza, Egypt
Email address

(R. M. Ismail)

(W. M. Raslan)

(G. M. H. Hussein)
Citation
Roba M. Ismail, Wesam M. Raslan, Gihan M. H. Hussein. In Vitro Propagation of Sugar Beet Cultivar Frida, Through Encapsulated Different Explants. American Journal of Agricultural Science. Vol. 3, No. 3, 2016, pp. 27-34.
Abstract
Synthetic or artificial seed protocol is a powerful method for production of seed analogues. It relies on in vitro encapsulation of any meristematic tissue by its coating with suitable gelling agents. Here we describe the synthetic seeds production for sugar beet (Beta vulgaris) cv Frida using encapsulation by sodium alginate. Different factors affecting the encapsulation efficiency such as explant type, presence of BA and presence of sucrose were studied. The best combinations for synthetic seed production found to be using of 4% sodium alginate for encapsulation of shoot tip explants in presence of 1.3 mg/l BA or 4% sucrose. In case of adventitious shoots, the best combinations found to be using of 4% sodium alginate with 1.3 mg/l BA, 4% sucrose and 1.2% agar.
Keywords
1. Introduction
Sugar beet (Beta vulgaris) is an important root crop and it considers the main source for sugar production in the moderate climate regions with five months long growing season [1]. In Egypt, the total planted sugar beet area in 2014/2015 is about 183,000 ha, comparing to 174,000 ha in 2013/14 as reported by Sugar Annual, 2015 (http://www.thefarmsite.com/reports/contents/EgyptSugar29April2014.pdf).
According to in vitro culture and genetic transformation, sugar beet considers as an unruly plant species [2]. Regenerated adventitious shoot from different explants has been effectively used for the propagation of elite sugar beet genotypes [3], although high degree of variability in the regeneration frequencies from diverse explants of different genotypes have been detected [4]. Additionally, the vegetative nature of the material and the greater risks of disease transfer increase the difficultly of the distribution and replace from gene bank field.
Artificial seed protocol extends plant biotechnology and agriculture prospect in the plant propagation [5] as it useful for protecting the best agricultural and endangered plant species, which are difficult to regenerate through conventional methods and natural seeds. In addition, artificial seed is very useful way for the large-scale propagation of superior hybrids of economically important species [6].
Encapsulated somatic embryo firstly reported by Murashige [7]. Afterward, engineering of somatic embryo into synthetic seed has been continued by the efforts of Kitto and Janick [8], Gray [9] and Gray and Purohit [10]. Encapsulated micro shoots or somatic embryos were used to propagate different plant species, such as sandalwood, mulberry, banana, cardamom, sugar beet, rice and peer [11, 12, 13, 14, 15, and 6]. Different types of the explants have been previously used to produce synthetic seeds such as nodeal segment from Bacopamonnieri L [16] and Menthaarvensis [17]; or somatic embryos and shoot tip from sugar beet [14, 18], Begoniaxhiemalis Forch [19] and shoot tip M. arvensis [17] and Stevia [20]. In general, the synthetic seed was used in propagating different economically important plant species such as vegetable crops, forage legumes, industrially important crops, cereals, spices, plantation crops, fruit crops, ornamental plants, orchids, medicinal plants and wood yielding forest trees [21].
In this work, synthetic seeds were produced from inter node, shoot tip explants and adventitious shoots from sugar beet cv. Frida and their ability to regenerate in complete plants was assessed. In addition, adventitious shoots development from the internode explants was studied.
2. Materials & Methods
2.1. Materials
Seeds of sugar beet (Beta vulgaris) cultivar Frida were kindly obtained from sugar crops institute, Agricultural Research Center (ARC).
2.2. Methods
Seeds germination
Sugar beet seeds were surface sterilized by soaking in 70% (v/v) ethanol for 1 min; then rinsed several times with sterilized distilled water. Seeds were transferred to 40% (v/v) Clorox solution for 45 min, then washed by rinsing several times with sterile distilled water for 30 min. Thereafter, seeds were germinated on MS medium supplemented with 0.5 mg/l 2, 3, 5-Triiodobenzoic Acid (TIBA) medium and incubated for 45 days.
2.3. Synthetic Seeds of the Shoot Tip and Internode Cutting Explants
2.3.1. Explants Encapsulation
I. Coating explant with alginate
Shoot tip and internode cutting as explants were coated with six coating solutions composed 4% sodium alginate dissolved in water or in MS basal medium with or without benzyl adenine (BA) and sucrose (Table 1 from T1-T6). Explants were coated by dropping the explants in sodium alginate solutions for 20 min; the alginate-coated explants were transferred to plates containing 1.4% CaCl2 for another 20 min. Then, coated explants were washed with liquid MS medium for three times. All procedure steps were conducted under aseptic condition.
II. Synthetic seeds storage
Coated explants were placed on 3.5 cm Petri dishes either on filter paper or without filter paper and incubated for 1, 2, 4 and 8 weeks at room temperature. To select the best coating condition, in vitro germination of coating seeds was performed on half SH9 medium [22]. The following parameters were recorded: Required time for germination in days, percentage of germination, number of shoots/explant, shoots height and root formation.
2.3.2. Shoot Development
For producing microshoots, the inter node segments were cut from 45-day-old in vitro seedling as explants, then cultured on different multiplication media composed of different media, different growth regulators and different solidifying agent (phytagel and agar, Table 2). Each multiplication medium had six plates on each five explants with total number of 30 explants. Obtained micro-shoots were isolated from their explants and were coated for producing synthetic seeds.
2.3.3. Encapsulated Microshoots
Obtained shoots were coated with two sodium alginate solutions (Table1, T7 and T8). Then the coated explants were then incubated into CaCl2, and washed as mentioned before with the shoot tip and inter-node explants.
2.3.4. In vitro Germination
To select the best germination medium encapsulated shoots were transferred to four different media which are: G1 (hormone-free MS medium); G2 (MS with 0.5 mg/l TIBA); G3 (MS with 40µg NAA) and G4 (half strength SH medium with 40 µg/l NAA). All germination media were solidified with agar at concentration of 8 g/l. Germinated sugar beet seeds were transferred to root formation medium containing 1mg/l IBA. The rooted plantlets were acclimatized on mixture of beet moss: sand (1:1).
Table 1. Different sodium alginate solution.
| Treatments | Sodium alginate | agar | MS | BA(mg) | sucrose |
| T1 | 4% | | + | | |
| T2 | 4% | | | 1.3 | |
| T3 | 4% | | + | | 4% |
| T4 | 4% | | + | 1.3 | |
| T5 | 4% | | | | |
| T6 | 4% | | | | 4% |
| T7 | 4% | 1.2% | + | | 4% |
| T8 | 4% | 1.2% | + | 1.3 | 4% |
Table 2. Different shoot development media composition.
| Media | medium | Growth regulatorsmg/l | Solidifying agentg/l |
| MS | SH | NAA mg/l | TIBA | BA mg/l | TDZ | Phytagel | Agar |
| SD1A | half | | | | 3 | | | 7 |
| SD2A | half | | | | | 3 | | 7 |
| SD3P | full | | | | 3 | | 3 | |
| SD3A | full | | | | 3 | | | 7 |
| SD4P | full | | | | | 3 | 3 | |
| SD4A | full | | | | | 3 | | 7 |
| SD5P | full | | | 0.5 | | | 3 | |
| SD5A | full | | | 0.5 | | | | 7 |
| SD6P | full | | 0.3 | | | | 3 | |
| SD6A | full | | 0.3 | | | | | 7 |
| SD7P | | half | | | | | 3 | |
| SD7A | | half | | | | | | 7 |
| SD8P | | half | | | | 3 | 3 | |
| SD8A | | half | | | | 3 | | 7 |
| SD9P | | half | | | 3 | | 3 | |
| SD9A | | half | | | 3 | | | 7 |
| SD10P | | half | 0.3 | | | | 3 | |
| SD10A | | half | 0.3 | | | | | 7 |
2.3.5. Root Formation
The obtained shoots were transferred to two different rooting media composed of MS with different concentration of Indole-3-butyric acid(IBA) (1 or 2 mg /l), individually.
2.3.6. Acclimatization Stage
Plantlets were transferred to greenhouse in pots containing 1:1 mixture of peat moss: sand. The pots were covered with transparent plastic bags to keep the humidity at 90% for one week. The plastic bags were removed gradually.
2.4. Synthetic Seeds of the Adventitious Shoots
Synthetic seeds were also produced from adventitious shoots that were developed from the inter-node explants.
3. Results
3.1. Synthetic Seeds of the Shoot Tip and Internode Cutting Explants
Explants (shoot tip & internode cutting) encapsulation:
In order to select the best solution of encapsulation, the encapsulated explants (synthetic seeds) were germinated after different storage periods. All coated explants from different coating treatment were germinated on half SH9 medium. Results showed that all explants incubated on a filter paper were dried after one weak. All encapsulated explants with T1, T2, T5 and T6 coating solutions did not germinate. While, the encapsulated shoot tip explants with T3 and T4 germinated easily. The internode coated explants with T3 or T4 that stored for one week, two weeks and a month germinated but those stored for two months did not germinate (Table 3). It was also showed that there were approximately no differences of the germination rate between the coating solutions (T3 and T4). All coated explants that were stored for one week started to germinate after three days, whereas, the encapsulated explants that incubated for two weeks started to germinate after four days. In addition, the germination time was varied from 6-12 days for the coated explants that incubated for one month. Furthermore, the internode coated explants incubated for two months did not germinate while the shoot tip coated explants incubated for two months were germinated after 7 days. The germination percentage of synthetic seeds was varied among the treatments from 0-100 (Table 3). Shoot heights were ranged 8.5-13 cm among the treatments. The highest germination percentage was in the synthetic seeds incubated for one week, while the lowest germination percentage was in the encapsulated explants incubated for two month. Thus shoot tip as an explant and T3 or T4 coating solutions reveled the best condition for producing synthetic seeds.
Not all treatments formed root on the half SH9 medium therefore the shoots were rooted on medium containing IBA (Fig. 1). It was observed that medium containing IBA at concentration of 1 mg/l was better than that containing 2mg/l (Data not shown).
Table 3. Germination of both encapsulated explants (shoot tip-inter node) with T3 & T4 solution.
| explant | Coating
treatment | Storage
time | Total No.
coating seeds | Germinating
seeds | % Germinating
seeds | Time of
germination | Obtaining shoots | Shoot heights par cm |
| Shoot tip | T3 | week | 15 | 15 | 100 | 3days | 7 | 13 |
| Two weeks | 15 | 11 | 76 | 4 days | 7 | 10 |
| month | 15 | 5 | 30 | 6 days | 2 | 10 |
| Two moths | 15 | 3 | 20 | 7 days | 1 | 9 |
| T4 | week | 15 | 15 | 100 | 3 days | 6 | 12 |
| Two weeks | 15 | 12 | 80 | 4 days | 7 | 10 |
| month | 15 | 5 | 33.3 | 7 days | 2 | 9 |
| Two moths | 15 | 3 | 20 | 7 days | 1 | 8.5 |
| Inter node | T3 | week | 15 | 15 | 100 | 3 days | 10 | 11 |
| Two weeks | 15 | 12 | 80 | 4 days | 10 | 12 |
| month | 15 | 3 | 20 | 10 days | 1 | 8 |
| Two moths | 15 | 0 | 0 | 0 | 0 | 0 |
| T4 | week | 15 | 15 | 100 | 3 days | 12 | 13 |
| Two weeks | 15 | 15 | 100 | 4 days | 10 | 13 |
| month | 15 | 3 | 20 | 10 days | 3 | 11.5 |
| Two moths | 15 | 0 | 0 | 0 | 0 | 0 |

Fig. 1. Germination of encapsulated shoot tip (A) & interned segments (B).
A, Encapsulated shoot tips on germination medium after 5 days (1)and after 10 days (2); germinated shoots on rooting medium after 3 weeks (3) and sugar beet plant after 45 days under acclimatize condition.
B, Encapsulated inter-nod segments on germination medium after 5 days (1) and after 2 weeks; germinated shoots after 3 weeks incubation on rooting medium and sugar beet plant after 45 days under acclimatize condition
3.2. Synthetic Seeds of the Adventitious Shoots
3.2.1. Adventitious-Shoot Differentiation
Results showed that the average of developed shoots per each explants ranged from 1-1.5 shoots. Among all media, SD2A and SD1A revealed highest response of shoot development. Medium SD2A containing 3mg/l TDZ revealed higher percentage rather than SD1A that contained 3mg/l BA. Whereas, the medium SD8P revealed highest percentage of shoot development but it resulted in the highest vitrification percentage as well. Media composed of half SH media (SD7P-SD10A) had low frequency of shoot production except SD8P medium which contained TDZ at concentration of 3 mg/l. It was observed that the percentage of vitrification was ranged from 7-38% in shoots that produced on media solidified with phytagel (Table 4).
Shoots developed on medium containing MS medium and BA was earliest than all other media as shoots started to germinate within 4-7 days of culturing, while adventitious shoots on other media started to differentiate after 10 days. It was observed that shoots developed on MS medium were greener, stronger and taller than those that developed on SH media (Fig. 2). Thus, medium SD1A composed of half MS, 3mg/l BA and 8g/l agar was selected for developing adventitious shoots from sugar beet internodes.
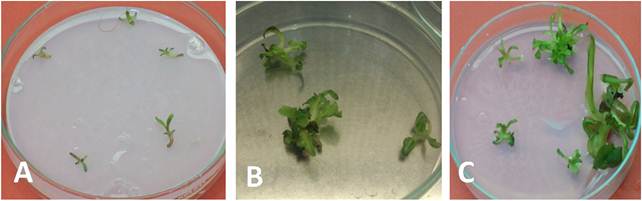
Fig. 2. Adventitious shoots differentiation on different media: obtained adventitious-shoots on half SH based medium (A), MS based medium (B). Difference on normal and vitrified shoots on medium solidified by phytagel (C).
Table 4. Effect of different media on shoot differentiation.
| Media | Total n of
explants | No of explants producing shoots | % explants
producing shoots | Total No of
shoots | Means of
shoot/explants | Number of Vitrified plants(v) | %(v) |
| SD1A | 30 | 30 | 100 | 39 | 1.3 | 0 | 0 |
| SD2A | 30 | 30 | 100 | 42 | 1.4 | 0 | 0 |
| SD3P | 30 | 30 | 100 | 37 | 1.2 | 13 | 35% |
| SD3A | 30 | 30 | 100 | 30 | 1.0 | 0 | 0 |
| SD4P | 30 | 30 | 100 | 42 | 1.4 | 15 | 36% |
| SD4A | 30 | 25 | 83 | 25 | 1 | 0 | 0 |
| SD5P | 30 | 30 | 100 | 30 | 1 | 9 | 30% |
| SD5A | 30 | 30 | 100 | 31 | 1 | 0 | 0 |
| SD6P | 30 | 30 | 100 | 30 | 1 | 6 | 20% |
| SD6A | 30 | 30 | 100 | 30 | 1 | 0 | 0 |
| SD7P | 30 | 28 | 93 | 28 | 1 | 10 | 36 |
| SD7A | 30 | 10 | 33 | 10 | 1.0 | 0 | 0 |
| SD8P | 30 | 30 | 100 | 45 | 1.5 | 17 | 38 |
| SD8A | 30 | 30 | 100 | 32 | 1.06 | 0 | 0 |
| SD9P | 30 | 30 | 100 | 30 | 1 | 2 | 7 |
| SD9A | 30 | 30 | 100 | 30 | 1 | 0 | 0 |
| SD10P | 30 | 20 | 66.6 | 20 | 1 | 3 | 15 |
| SD10A | 30 | 30 | 100 | 35 | 1.16 | 0 | 0 |
3.2.2. Explants Encapsulation
Obtained adventitious shoots were coated with two coating solutions composed of 4% sodium alginate, 1.2% agar and 4% sucrose (T7) or 4% sodium alienate, 1.2% agar, 4% sucrose and 1.3 mg/l BA (T8) dissolved into liquid MS medium. After one-week incubation at 25°C, the encapsulated explants were germinated on four different media. Seeds started to germinate after 3-4 days on all media; shoots reach 2-11 cm height during 3-4 weeks. From the data in Table (5), the best conditions for germination were at hormone free MS medium and T8 coating solution, because it counted the height seed germination, shoots and root formation (Fig. 2). The germinated shoots that did not form roots were transferred to root formation medium containing 1mg/l IBA (Fig. 3).
Table 5. Encapsulation adventitious shoot explant germination on different media.
| Germination media | Coating
treatment | Total No.
coating seeds | Germinating
seeds | % Germinating seeds | Obtaining
shoots | Shoot heights par cm | Root formation |
| G1 | T7 | 15 | 8 | 53.3 | 8 | 11 | 0 |
| T8 | 15 | 15 | 100 | 30 | 4 | 1 |
| G2 | T7 | 15 | 8 | 53.3 | 8 | 2 | 0 |
| T8 | 15 | 6 | 66.7 | 10 | 2.5 | 0 |
| G3 | T7 | 15 | 6 | 40 | 9 | 6.3 | 1 |
| T8 | 15 | 10 | 66.7 | 20 | 3.2 | 0 |
| G4 | T7 | 15 | 8 | 53.3 | 8 | 3.5 | 1 |
| T8 | 15 | 8 | 53.3 | 18 | 3.1 | 0 |

Fig. 3. Adventitious shoot explant before coating: Adventitious shoot used for coating (A); coated adventitious shoots with alginate/agar (B); coating shoots after one week incubation (C); germinated of synthetic seeds after 2 weeks on MS medium (D); Shoots after 3 weeks on rooting medium (E); acclimatized plants after 45 days (F) and after 100 days (G) and produced sugar beet tap-root after 100 days (H).
4. Discussion
The artificial seed technology has opened a new scope for handling, transplantations and maintenance of rare and attractive genotypes [23]. In this investigation the production of the synthetic seed from in vitro materials of sugar beet cv Freda was studied. Three sugar beet explants were used, i.e., shoot tip, internode cutting and adventitious shoots that were developed from the internode cutting explants. Several explants were used previously to produce synthetic seeds such as: the nodal segment in Bacopamonnieri L [16]; Cannabis sativa L. [24] and M. arvensis[17]; shoot tip of M. arvensis[17]; Stevia [20].Synthetic seeds of sugar beet were produced previously from somatic embryos [14] and shoot tip [17].
Kitto and Janick [25] used many encapsulating agents such as agar, agarose, alginate, carrageenan, gelrite and polyacrylamide. Alginate is one of the most commonly used polymers for immobilization of plant cells and production of synthetic seeds because it is available in large quantities, inert, non-toxic, cheap and can be easily handled [26,27]. Sodium alginate is recommended as most suitable encapsulating agent due to its solubility at room temperature and its ability to form completely permeable gel with calcium chloride forming a hydrogel in the presence of calcium ions [28,29]. In this study, sodium alginate has been used as an encapsulating agent for coating explants in different matrix solutions. It was found that the presence of MS, sucrose and BA in encapsulating solutions is important in the artificial seed germination. Results showed that all explants coated with sodium alginate solution did not germinate. Moreover, the synthetic seeds that coated with solution of sodium alginate and sucrose dissolved in water did not germinate. While, the synthetic seeds that coated with sodium alginate with sucrose dissolved in MS germinated. Explants coated with encapsulation solution containing 4% sodium alginate dissolved in MS medium with either 1.3 mg/l BA or 40 g/l sucrose revealed the same germination frequency. This indicates the importance of sucrose, BA or MS for synthetic seed germination. Furthermore, the adventitious shoot explants were coated with matrix solution that containing BA and sucrose revealed higher germination rate than those coated with solution contained only sucrose. This result indicates that the combination of BA and sucrose in coating solution affected positively on seed germination as well as the shoot development frequencies from the synthetic seeds. We concluded that the best coating (encapsulating) matrix solution was 4% sodium alginate, 1.2% agar as a solidified agent, 1.3 mg/l BA and 3% sucrose. The enhancement effect of sucrose in producing synthetic seeds was previously reported by Rizkalla et al., [18] while conducting the synthetic seeds production of sugar beet cvs Francesca and Toro by encapsulating shoot tip with matrix solution composed of 4% sodium alginate and 30 g/l sucrose. They found that the addition of sucrose at 30 g/l to encapsulation matrix solution gave high germination rate of synthetic seeds than addition of 40, 50 or 60 g/l sucrose. Tsvetkov et al. [30] and Maqsood et al. [31] reported that supplying 3% sucrose to the alginate solution was very important in the starting stage of the re-growth and synthetic seeds germination of hybrid aspen and Catharanthusroseus L, respectively. Murthy et al. [32] found that the shoot developing percentage, a high number of shoots obtained of each encapsulated explant and maximum shoot length were achieved with the encapsulation matrix prepared with MS supplemented with 3 mg/l BA, 3% sucrose and 3% sodium alginate in Ceropegiaspiralis. Our results showed also that the coating solution containing both sucrose and BA has also influenced positively on producing synthetic seeds from adventitious shoot explants as it reveled higher shoot development than the explants coated by solution without BA. Tsvetkov et al. [30] reported the positive effect of combining BA and sucrose in the coating solution. They observed that the highest fresh mass was shown when using MS media with either BA and sucrose or MS with only sucrose. They also demonstrated that BA increases the mass weight twenty fold. Contrarily, Nower [20] encapsulated stevia explants in MS liquid supplemented with only 4% sodium alginate. It was indicated that the encapsulation solution optimization is species-dependent and there is no general encapsulation solution for every plant.
Nature of explants was found to be an important factor in producing synthetic seed. The synthetic seeds from shoot tip explants were earlier in germination than those produced from internode. In addition, shoot tip synthetic seeds could be stored viable for two months while, the synthetic seed from inter node lost its germination ability after two months. This was agreed with Islam and Bari [17] as they reported that the artificial seed of shoot tip of M. arvensis exhibited highest percentage of multiple shoot formation than nodal segment explants.
The storage time of the synthetic seeds was also found to be affecting the required germination time. The required germination time was prolonged when the storage time was increased. It was three or four days in case of seeds stored for one or two weeks, respectively, and varied from 6-10 days in seeds that stored for month or two months. In our result only 4 days required to germinate the one week stored synthetic seeds on hormone free MS. Whereas, Sionget al. [6] reported that the artificial seeds produced from adventitious shoots of cauliflower, Brassica oleracea var. botrytis required 12 days (after 7 days storage) and 14 days (after 30 days storage) to germinate on MS basal medium.
Artificial seeds of sugar beet in current study were stored at empty Petri dishes, while Rizkalla et al. [18] stored their seeds on MS medium with sorbitol or mannitol.
5. Conclusion
In this study, synthetic seeds of sugar beet (Beta vulgaris) cv Frida from different explants were produced. Our established protocol showed efficient germination rate and storage time. We also concluded the importance of sucrose in the encapsulation by alginate.
References
- Trifonova, A. and A. Atanassov (1995). Genetic Transformation of Sugar Beet by AgrobacteriumRhizogenes. Biotechnology &Biotechnological Eq., 9:23-26.
- Krens, F.A.; A. Trifonova; L. C.P. Keizer and R. D. Hall (1996). The effect of exogenously-applied phytohormones on gene transfer efficiency in sugar beet (Beta vulgaris L.). Plant Sci., 116:97-106.
- Grieve, T.M.; K.M.A. Gartland and M.C. Elliott (1997). Micropropagation of commercially important sugar beet cultivars. Plant Growth Regul, 21:5-18.
- Saunders, J.W. and C.J. Tsai, (1999). Production of somatic embryos and shoots from sugar beet callus: Effects of abscisic acid, other growth regulators, nitrogen source, sucrose concentration and genotype. In Vitro Cell. Dev. Biol. Plant, 35:18-24.
- Kinoshita I.(1992).The Production and Use of Artificial Seed, Research Journal of Food and Agriculture, 15(3):6-11.
- Siong, P. K.; Sadegh M. and Rosna M. T. (2012). Production of Artificial seeds derived from encapsulated in vitro microshoots of cauliflower, Brassica oleracea var. botrytis Romanian, Biotechnological Letters, 17:4 (7549-7556).
- Murashige T.(1977). Plant cell and organ culture as horticultural practice. ActaHortic, 78:17-30.
- Kitto, S. L. and J. Janick, (1982). Polyox as an artificial seed coat for asexual embryos. Horticultural Science, 17:448.
- Gray, D. J. (1987). Synthetic seed technology for the mass cloning of crop plants: problems and prospects. Horti. Sci., 22:795-814.
- Gray, D. J. and A, Purohit. (1991). Somatic embryogenesis and development of synthetic seed technology. Crit Rev Plant Sci., 10:33-61.
- Bapat, B.A. and P.S. Rao (1998). Sandalwood plantlets from synthetic seeds. Plant Cell Rep., 7:434-436.
- Ganapathi, T. R.; P. Suprasanna; V. A. Bapat and P. S. Rao (1992). Propagation of Banana through enhanced shoot tips .Plant Cell Rep, 11:571-575.
- Ballester, A.; L.V. Janeiro and A. M. Vieitez (1997).Cold storage of shoot cultures and alginate encapsulation of shoot tips of Camellia japonica L. and Camellia reticulate Lindly. SciHortic, 7:67-78.
- Tsai, C. J.and J. W. Saunders (1999).Encapsulation, germination and conversion of somatic embryos in sugar beet. Journal of Sugar Beet Research, 36(4):11-32.
- Nower, A.A.; E A. Ali and A. A.Rizkalla (2007).Synthetic seeds of Pear (Pyruscommunis L.) Rootstock storage In vitro, Australian Journal of Basic and Applied Sciences, 1(3):262-270.
- Ramesh, M.; R. Marx; G. Mathan; S. K. Pandian (2009). Effect of bavistin on in vitro plant conversion from encapsulated uninodeal micro-cuttings of micro-propagated Bacopamonnieri (L) An Ayurvedic Herb. J. Environ. Biol., 30:441-444.
- Islam, M. S. and M. A. Bari (2012). In vitro regeneration protocol for artificial seed production in an important medical plant Menthaarvensis L. 20:99-108.
- Rizkalla, A. A.; A. M. Badr-Elden; M. E. Ottai; M.I. Nasr and M. N. M. Esmail (2012). Development of artificial seed technology and preservation in sugar beet. Sugar Tech., 14(3):312-320.
- Awal, A.;R. M.Taha and N. A. Hasbullah (2007).In vitroformation of synthetic seeds of Begoniaxhiemalis Fotch. Indian J. Environ. Sci. 2:189-192.
- Nower, A. A.(2014). In Vitro Propagation and Synthetic Seeds Production: An Efficient Methods for Stevia rebaudiana Bertoni, Sugar Tech, 16(1):100-108.
- Reddy, M. C.; K.S. R. Murthy and T. Pullaiah (2012). Synthetic seeds: A review in agriculture and forestry, African J. of Bio. 11(78), 14254-14275.
- Trinh, T. H.; P. Ratet; E. Kondorosi; P. Durand; K. Kamaté; P. Bauer and A. Kondorosi (1998). Rapid and efficient transformation of diploid Medicago truncatula and Medicago sativa ssp. falcata lines improved in somatic embryogenesis. Plant Cell Rep., 17:345-355.
- Zhang, Y. F., S. Yan and Y. Zhang (2011). Factors affecting germination and propagators of artificial seeds of Dendrobium Candidum. International Conference on Agricultural and Biosystems Engineering. Advances in Biomedical Engineering, (1-2):404-410.
- Lata, H.; S. Chandra; T. Natascha; I. A. Khan and M. A. ElSohly (2011).Molecular analysis of genetic fidelity in Cannabis sativa L. plants grown from synthetic (encapsulated) seeds following in vitro storage. Biotechnol. Lett. 33:2503-2508.
- Kitto, S. L.and J. Janick (1985). Production of synthetic seeds by encapsulating asexual embryos of carrot. J Am Soc Hortic Sci, 110:277-282.
- Endress R. (1994). Plant Cell Biotechnology. Springer-verlag, Berlin. 256-269.
- Jaiswal, V.S.; A. Hussain and U. Jaiwal (2001). Synthetic seed: Prospects and limitations. Current Science, 78(12):1438-1444.
- Bapat, V.A.; M Mhatre and P.S. Rao (1987). Propagation of Morusindica L. (Mulberry) by encapsulated shoot buds. Plant Cell Rep., 6, 393-395.
- Kikowska, M. and B. Thiem (2011).Alginate-encapsulated shoot tips and nodeal segments in micropropagation of medicinal plants. A review 57 (4).
- Tsvetkov, I; L.Jouve and J. F. Hausman (2006).Effect of alginate matrix composition on re-growth of in vitro-derived encapsulated apical microcuttings of hybrid aspen, Biologia Plantarum, 50 (4):722-724.
- Maqsood, M.; A. Mujib and Z.H. Siddiqui (2012). Synthetic seed development and conversion to plantlet in Catharanthusroseus(L.) G. Don. Biotechnology, 11:37-43.
- Murthy, K. S. R.; M. C. Reddy and R. Kondamudi (2013). Synthetic seeds – A novel approach for the conservation of endangered C. spiralis wt. and C. pusilla Bangladesh J. Sci. Ind. Res. 48(1), 39-42.

 (R. M. Ismail)
(R. M. Ismail)  (W. M. Raslan)
(W. M. Raslan)  (G. M. H. Hussein)
(G. M. H. Hussein) 




