Evaluation of Phyto-Chemical Remediation Approaches to Remedy Hydrocarbon from Oil Polluted Soils and Their Impact on Soil Microbial Communities Using RAPD and ISSR Markers
Shreen S. Ahmed1, Mohamed A. M. Atia2, *, Gehan H. Abd El-Aziz1, Ashraf H. Fahmy3
1Soils, Water and Environment Research Institute, ARC, Giza, Egypt
2Genome Mapping Department, Agricultural Genetic Engineering Research Institute, ARC, Giza, Egypt
3Plant Genetic Transformation Department, Agricultural Genetic Engineering Research Institute, ARC, Giza, Egypt
Email address

(M. A. M. Atia)
*Corresponding author
Citation
Shreen S. Ahmed, Mohamed A. M. Atia, Gehan H. Abd El-Aziz, Ashraf H. Fahmy. Evaluation of Phyto-Chemical Remediation Approaches to Remedy Hydrocarbon from Oil Polluted Soils and Their Impact on Soil Microbial Communities Using RAPD and ISSR Markers. American Journal of Agricultural Science. Vol. 3, No. 3, 2016, pp. 48-58.
Abstract
Soil contamination by petroleum hydrocarbons is one of the world’s most common environmental problems. Remediation of the petroleum contaminated soil is essential to maintain the sustainable development of soil ecosystem. In this study, we evaluate the efficiency of different Phyto-chemical approaches for cleaning up hydrocarbon contaminated soils and their effect on the soil properties, soil microbial communities structure, grain yield, chemical composition of wheat plants (Triticum aestivum L). The experiment included five treatments: phytoremediation (Phyto) and Phyto combination with organic and inorganic compound. The degradation rate of total petroleum hydrocarbons (TPHs) was in the following ascending order: Phyto + nitrogen (16.7%), phytoremediation (40.0%), Phyto + potassium permanganate (61.5%), Phyto + bacteria (63.7%), Phyto + humic acid (76.0%). Results revealed that yield, protein, fat, macronutrients contents were decreased whereas; carbohydrate was increased as applied of TPH in the soil compare to the control. Results also revealed that wheat grain that grown in contaminated soil (Phyto) had higher concentrations of total petroleum hydrocarbon compare to unpolluted soil (control) and Phyto combinations with organic and inorganic compound treatments. It can be concluded that Phyto combination with humic acid, bacteria and potassium permanganate was more effective for cleaning up hydrocarbon contaminated soils than phytoremediation treatment separately. On the other hand, Randomly Amplified Polymorphic DNA (RAPD) and Inter-simple sequence repeats (ISSR) molecular marker systems were used to survey and explore the diversity of soil microbial communities under different Phyto-chemical treatments. Cluster analysis based on combined data of RAPD and ISSR fingerprinting was discussed. The molecular phylogeny results exhibited the ability to differentiate and track genetic variations in bacterial populations. Such approaches represent a fundamental step for studying structure and dynamics of microbial communities in contaminated ecosystems.
Keywords
Phytoremediation, Phyto-chemical Remediation, Petroleum Hydrocarbons, RAPD, ISSR, Soil Microbial Communities
1. Introduction
Soil contamination by petroleum hydrocarbons is one of the world’s most common environmental problems [1]. Total petroleum hydrocarbons (TPHs) are one of the most common groups of persistent organic contaminants [2]. Generally, the accumulation of contaminants in soils can have destructive effects on both soil ecosystem and human health. Contaminants present in soils can enter the food chain and seriously affect animal and human health [3]. In today’s era of heightened environmental awareness and good stewardship of limited natural resources effort to clean up contaminated sites involve series of remedial techniques or approaches ranging from conventional physicochemical techniques and natural attenuation to phytoremediation the most emerging biotechnology approach [4]. Phytoremediation is one of the best developed and implemented approaches/technologies of bioremediation for cleaning up the environmental pollution. Phytoremediation has been proposed as a cost effective, non-intrusive, and environmental friendly technology for the restoration of soils contaminated with TPH [5].
Studying of the structure and dynamics of an ecosystem is used as an indicator to measure the cumulative impact of multiple stresses on population (s) and its adaptation to the habitat. Microbial communities, capable of degrading different pollutants in contaminated ecosystems, are relevant in microbial ecology for the development of bioremediation strategies. Analysis of biodiversity is particularly important when the soil ecosystems respond to changing environmental conditions and such changes in the composition of the soil micro-flora can be crucial for the functional integrity of the soil as a main component in agriculture system. In recent years, several studies were performed to describe bacterial diversity and community changes in various pollutant-degrading communities [6, 7, 8] and a number of molecular methods have been developed for describing and comparing complex microbial communities [9]. Polymerase chain reaction (PCR) has been successfully used for microbial identification in the environmental context.
PCR-Based molecular markers have been potentially used to survey and explore the diversity of soil microbial communities, bacterial taxonomy and phylogeny. Randomly amplified polymorphic DNA (RAPD) and Inter-simple sequence repeats (ISSR) based detection of genetic polymorphism has been successfully utilized to identify isolates, genetic diversity and population structure of bacteria; to demonstrate genetic variation within the species; and to elucidate the distribution of genes and population structure of the species [10]. RAPD markers have also been utilized for inter-specific and intra-specific genetic diversity of microbial communities of the soil across different treatments [11].
Therefore, this study aims to: (1) evaluate and compare the efficiency of different Phyto-chemical approaches for cleaning up hydrocarbon contaminated soils (2) explore the genetic diversity between microbial communities of different Phyto-chemically treated soils using RAPD and ISSR markers.
2. Materials and Methods
2.1. Soil Used for Experiment
Unpolluted surface soil (0-25 cm) was collected from an Agricultural Research Station, Giza. The soil was air dried and ground to 20 meshes before used. Spent engine oil was then added to a portion of the unpolluted soil with a dosage of 2% of soil mass.
2.2. Experimental Design
A greenhouse experiment was carried out to study the effect of different Phyto-chemical remediation treatments on the spent engine oil contaminated soil. Seven treatments were designed:
1). Control (unpolluted soil with wheat planting).
2). Phytoremediation (Phyto; polluted soil with wheat planting).
3). Phytoremediation + Potassium permanganate (PK) addition to polluted soil (rate 0.9 M/Kg) (Phyto + PK).
4). Phytoremediation + Nitrogen addition to polluted soil (rate 0.2 g/kg) (Phyto + N).
5). Phytoremediation + Humic Acid (HA) addition to polluted soil (rate 15 g/kg) (Phyto + H).
6). Phytoremediation + Pseudomonas aeruginosa bacteria addition (Phyto + B; the soil was enriched with 30ml of bacterial suspension of Ps. aeruginosa incubated for 48 h at 28°C in 0.9% NaCl solution) [12].
Three replicates were sown for each treatment. Four Kilograms crude oil contaminated soil was added. For wheat (Triticum aestivum L.) planting, 10 seeds were sown evenly to the soil in each pot and covered with 2–3 cm of soil on the top (4 Kg soil added in total for each pot). Pots were irrigated every day of field capacity. All of the experiment pots were placed in a greenhouse at 30°C. Seven days after seeds germinated, 5 healthy seedlings were preserved in each pot for further remediation. Soil samples were taken after 0, 15 30, 45, 60, 75, 90, 105 and 120 days. Then the soil samples were divided into two sub samples: one was used to study the variations of soil physicochemical properties and evaluation of hydrocarbon degradation.
2.3. Analytical Methods
Some Physical and chemical characteristics of the studied soil was determined according to Page et al. [13]. Total petroleum hydrocarbon were extracted from soil and plant samples then determined by UV-Spectrometer according to the procedure described by IOC [14]. The extracts were analyzed using a Hewlett Packard (HP) 5890 Series II gas chromatograph (GC) with a 5971A mass selective detector (MSD), a HP 7673 autosampler, and HP Chemstation software. The instrument was operated in the splitless mode with 1 µL injections onto a 30 m x 0.25 mm x 0.25 µm RTX-5 (5% phenylmethylsiloxane) capillary column. The run time to elute all the target compounds was about 35 minutes, but the full cycle time was about 60 minutes. In plant sample, phosphorus content was determined by vanadomolybdate yellow method spectrophotometrically and potassium by flame photometer [15]. Heavy metal contents samples were extracted according to the method of Lindsay and Novell [16]. Total nitrogen was determined by micro-Kjeldahl method according to AOAC [17]. Crude protein was calculated by multiplying the values of total nitrogen in 6.25. Total lipid was determined according to AOAC [18]. Total carbohydrate was extracted according to Smith et al. [19] and determined using spectrophotometer according to Murphy [20]. All data were statistically analyzed using Costat computer program according to procedures outlined by Snedecor and Cochran [21].
2.4. Soil Sampling and Estimation of Microbial Community
Soil samples were collected from both control and treatments, and then stored at 4°C till DNA isolation and molecular analysis. For each sample (control and treatments), 10 g was suspended into 90 mL of sterile doubled distilled water (ddH2O) and stirred using shaker at 200 rpm for 1 hour. The slurry was centrifuged at 5000 rpm for 5 min at 25°C. Then, 1 ml of the supernatant was used to inoculate 100 mL of LB broth medium and incubated at 37°C for 24 h for microbial growth. The number of viable cells was determined by serial dilution technique and spectrophotometry as indirect approach to estimate the microbial community’s variations between control and treatments [22].
2.5. Bacterial DNA Isolation
The bacterial chromosomal DNA was extracted from whole bacterial community (Gram Positive and Gram Negative Bacteria) using Wizard Genomic DNA Purification Kit (Promega, WI, USA) according to manufacturer instructions. The DNA was quantified with NanoDrop Spectrophotometer (Thermo Fisher Scientific Inc.). All samples were adjusted to a concentration of 10 ng/µl for subsequent molecular analyses.
2.6. RAPD Marker Analysis
Ten RAPD decamer primers (Operon Tech., Alameda, CA, USA) were used in the present study. DNA was amplified following the protocol of Adawy and Atia [23]. Each PCR reaction mix of 25 μl contained the 30 ng template DNA, 2.5 μl of 10X PCR buffer, 1.5 μl of 25mM MgCl2, 2.5 μl of the dNTPs mix, 30 pmol of RAPD primer, 1.0 U Taq DNA polymerase (Promega, WI, USA). The amplification was performed in a thermal cycler (Applied BioSystems, USA) programmed for initial denaturation of 5 min at 94°C; 40 cycles of 2 min denaturation at 94°C, 1 min annealing at 36°C and 2 min extension at 72°C; and final elongation step at 72°C for 7min. The PCR products were electrophoresed on 1.5% agarose gel containing ethidium bromide (0.5 μg/mL) in TBE buffer for 2 h at 100 V. After electrophoresis, the gels were observed under an UV-transilluminator, documented in Gel-Doc XR (Bio-Rad) and photographed. The size of the amplicons was determined using 100 bp DNA ladder plus.
2.7. ISSR Marker Analysis
Five ISSR primers were used in the present study. DNA was amplified according to the following protocol. Each PCR reaction mix of 25 μl contained the 30 ng template DNA, 2.5 μl of 10X PCR buffer, 1.5 μl of 25mM MgCl2, 2.5 μl of the dNTPs mix, 30 pmol of ISSR primer, 1.0 U Taq DNA polymerase (Promega, WI, USA). The amplification was performed in a thermal cycler (Applied BioSystems, USA) programmed for initial denaturation of 5 min at 94°C; 40 cycles of 2 min denaturation at 94°C, 45 Sec. annealing at 50°C and 2 min extension at 72°C; and final elongation step at 72°C for 7 min. The PCR products were electrophoresed on 1.5% agarose gel containing ethidium bromide 0.5 μg/ml in TBE buffer for 2 h at 100 V. After electrophoresis, the gels were observed under an UV-transilluminator, documented in Gel-Doc XR (Bio-Rad) and photographed. The size of the amplicons was determined using 100 bp DNA ladder plus.
2.8. Markers Data Analysis
The generated/ amplified bands were scored visually. The bands were scored as present (1) or absent (0) to create the binary data set. To estimate the genetic similarity, Jaccard’s coefficient was used [24]. A dendrogram was generated by cluster analysis using the un-weighted pair group method of the arithmetic averages (UPGMA) using SPSS program V1.6. Support for clusters was evaluated by bootstrapping analysis. One thousand permutation data sets were generated by re-sampling with replacement of characters within the combined 1/0 data matrix.
3. Results
3.1. Effect of Spent Engine Oil on Soil Properties
The physicochemical properties of the tested soil shown in Table 1. Chemical properties of spent engine oil are presented in Table 2.
Soil physicochemical analysis was done after the addition of spent engine oil as source of hydrocarbon (PHCs). Results of the physicochemical analysis of the soil samples were reported in the table (3). Results of the pH revealed that the pH of the polluted soil sample was slight decrease compared to unpolluted soil as a control. The results of the organic carbon revealed that there was progressive increase in organic matter of polluted soil; increase reached 4.6 fold than in unpolluted soil as a control. Similar trend was found in the conductivity (EC) of the soils; the conductivity value of the soil after polluted was found maximum (2.98) and minimum (1.78) in control. Data of moisture content of oil polluted soils were lower than the control sample, decrease reached 54%. The bulk density of spent engine oil treated soil generally increased compared to unpolluted soil, increase reached 8.5%. The increase in bulk density of spent engine oil treated soil could be attributed to compaction resulting from oil contamination as well as reduced porosity. Also, data of the some macro, micronutrient and heavy metals revealed that there was increase in metals concentrations of polluted soil relative to control (unpolluted soil).
Table 1. Some and chemical properties of the tested soil under different experiments.
| Physical properties | Value |
| Coarse sand% | 7.3 |
| Fine sand% | 19.9 |
| Silt% | 38.3 |
| Clay% | 34.5 |
| Texture soil | loamy clay |
| Chemical properties | |
| pH (1: 2.5, soil - water suspension) | 7.99 |
| Organic matter (%) | 1.15 |
| Ece dS m-1, soil paste extract | 1.78 |
| Soluble cations (me/L) | |
| Ca++ | 7.1 |
| Mg++ | 3.9 |
| Na+ | 5.1 |
| K+ | 1.5 |
| Soluble anions (me/L) | |
| CO3= | - |
| HCO3- | 4.1 |
| Cl- | 7.1 |
| SO4= | 6.4 |
Table 2. Chemical properties of spent engine oil.
| Parameter | Oil |
| Organic carbon% | 5.98 |
| Total nitrogen% | 2.0 |
| K% | 0.98 |
| Na% | 0.81 |
| P% | 0.26 |
| Mg (mg/kg) | 1.7 |
| Ca (mg/kg) | 170 |
| Mn (mg/kg) | 0.7 |
| Fe (mg/kg) | 65.9 |
| Co (mg/kg) | 0.3 |
| Ni (mg/kg) | 50.8 |
| Cu (mg/kg) | 2.7 |
| Zn (mg/kg) | 19 |
| Cd (mg/kg) | 2.3 |
| Pb (mg/kg) | 105.3 |
Table 3. Comparison of spent engine oil polluted and unpolluted soils before planting.
| Parameter | Polluted | Unpolluted |
| Moisture content (%) | 3.19 | 6.93 |
| Bulk density (gcm-3) | 1.40 | 1.29 |
| ECe dS m-1, soil paste extract | 2.98 | 1.78 |
| pH | 7.68 | 7.99 |
| NH4 (ppm) | 99.4 | 49.4 |
| NO3 (ppm) | 49.7 | 39.76 |
| Organic carbon (%) | 7.66 | 1.68 |
| Total N (%) | 2.1 | 0.19 |
| P % | 1.28 | 1.02 |
| K % | 1.33 | 0.44 |
| Na % | 1.3 | 0.49 |
| Ca (mgkg-1) | 186 | 16 |
| Mg (mgkg-1) | 8 | 6 |
| Heavy metals |
| Mn+2 | 9.88 | 9.18 |
| Fe+2 | 625.9 | 560 |
| Co+2 | 0.615 | 0.382 |
| Ni+2 | 51.7 | 0.680 |
| Cu+2 | 7.51 | 3.9 |
| Zn+2 | 29 | 10.6 |
| Cd+2 | 2.5 | 0.2 |
| Pb+2 | 108 | 2.86 |
| TPH (mgkg-1) | 17610 | 104 |
3.2. Effects of Remediation Treatments on Hydrocarbon Degradation
Five different treatments (Phyto, Phyto + N, Phyto + PK, Phyto + HA and Phyto + B) were individually used for degradation of soil polluted with TPH compared with control. The degradation rate of hydrocarbon (fig. 1) using addition of different treatments was in the following ascending order: Phyto + N (16.7%), Phytoremediation (40.0%), Phyto + PK (61.5%), Phyto + B (63.7%), Phyto + HA (76.0%). On the other hand, the effect of HA or B or PK without Phyto on TPH degradation was calculated and recorded as 36, 23.7 and 21.5%, respectively. HA and B addition with Phyto significantly stimulated the degradation of hydrocarbon at the initial time.
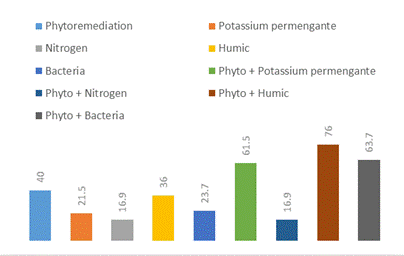
Figure 1. Effect of different treatments on hydrocarbon degradation% in soil.
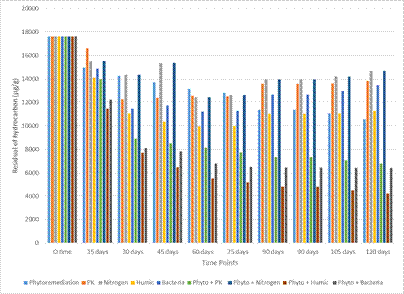
Figure 2. Interaction effect of different treatment and time on residual hydrocarbon in soil.
Data of residual hydrocarbon are presented in figure (2). Data showed that all treatments under investigation decreased strongly hydrocarbon concentration. Percentages of residual hydrocarbon at the end time of experiment (120 days) reached 59.9, 38.5, 23.9, and 36.3%, respectively. It worth mention, Phytoremediation combined with HA, B and PK was more effective for cleaning up hydrocarbon contaminated soils than phytoremediation individually. The hydrocarbon degradation efficiency of Phyto + HA treatment was more effective than others. At the end time (120 days), the hydrocarbon degradation rate increased at different degrees under different treatments compared with the control.
3.3. Effect of Spent Engine Oil on Growth and Chemical Composition of Wheat Plants
Results of the study showed that there was significant difference in the chemical composition of the wheat grain grown in the polluted soil, and those grown in the unpolluted soil. Wheat grown in spent engine oil treated soil (Table 4) recorded the lowest dry weight which was significantly different (P<0.05) from that of the control. Decline percent reached 53.4 and 48.6% for plant and grain dry weight, respectively. On the other hand, wheat plants grown in spent oil polluted soils with addition of HA recorded the highest dry weight which was significantly different from the other treatments. However, plants in the control experiments recorded the highest dry weight.
Protein and fat content grain of wheat grown in the control experiment (unpolluted soil) recorded the highest values. The values were significantly different from that of the other treatments. On the contrary, grain of wheat plants grown in the spent oil polluted soils produced (Phyto) the lowest values of protein and fat (Table 4). HA treatment recorded the highest values in protein and fat followed with B and PK treatments. Protein and fat contents were observed to be higher in the wheat plants grow in the control experiment (unpolluted soil). In contrast, percentage of protein and fat was decreasing in the grain of the treated wheat, as the addition of the spent oil (Phyto). Concerning the total carbohydrates (Table 4), data indicated that grain of wheat that grown in the spent oil polluted soils recorded the highest value compared to control (unpolluted) and other treatments. Treatments of HA and B were recorded the same trended. Further, PK treatment recorded the higher value of carbohydrate than HA and B treatments.
Table 4. Effect of spent engine oil pollution on chemical composition % of wheat grains.
| Treatment | Dry weight (g/pot) | Carbohydrates% | Fat% | Protein% |
| Plant | grain |
| Control (unpolluted soil) | 13.10A | 6.17A | 67.47D | 1.54A | 11.33B |
| Phytoremediation | 6.33E | 3.17E | 72.50A | 1.02E | 10.63C |
| Potassium permanganate | 8.00D | 3.60D | 69.43B | 1.22D | 11.62B |
| Humic acid | 9.40B | 5.23B | 68.17C | 1.40B | 12.86A |
| Bacteria | 8.43C | 4.07C | 68.60C | 1.32C | 11.61B |
| LSD at 0.050 | 0.1191 | 0.1031 | 0.6440 | 0.0595 | 0.6101 |
Table 5. Effect of spent engine oil pollution on macronutrients % of wheat plant and soil after harvesting.
| Treatment | Plant | Soil |
| N | P | K | N | P | K |
| Control (unpolluted soil) | 1.76B | 0.23A | 0.40B | 0.10C | 0.41D | 0.32C |
| Phytoremediation | 1.66C | 0.16B | 0.36B | 0.41B | 1.12A | 0.97B |
| Potassium permanganate | 1.81B | 0.20AB | 0.51A | 0.39B | 0.97C | 1.18A |
| Humic acid | 2.00A | 0.21AB | 0.45AB | 1.60A | 1.12A | 0.95B |
| Bacteria | 1.81B | 0.21AB | 0.43AB | 0.36B | 0.98BC | 0.93B |
| LSD at 0.050 | 0.10 | 0.06 | 0.10 | 0.15 | 0.06 | 0.06 |
3.4. Effect of Spent Engine Oil Pollution on Macronutrients of Wheat Plant and Soil After Harvesting
Data of the effect of spent engine oil pollution on macronutrients of wheat grain and soil are presented in Table (5). Macronutrient contents (Nitrogen, phosphorus, and potassium) of wheat grown in the unpolluted soil recorded the higher values than macronutrient contents of wheat plants grown in the spent oil polluted soils. Decline percent reached 5.68, 30.4, and 10.0%, respectively. On the other hand, significant effect was observed when wheat plants grown in spent oil polluted soils with addition of different treatments.
3.5. Content of Hydrocarbons by Wheat Grown Under Different Treatments
Content of TPH by wheat grown in different field-contaminated soils was investigated. TPH concentrations in grain correlated positively with the corresponding concentrations in soils (Figure 3). Result of the experiment indicated that wheat grain that grown contaminated soils (phyto) had higher concentrations of total petroleum hydrocarbon compare to unplanted soil (control). Increase percent reached 80.6% related to unpolluted soil. On the other hand, progressive effect was observed when wheat plants grown in spent oil polluted soils (phyto) with addition of different treatments (PK, HA, and B). All treatments recorded the lower values in hydrocarbon contents than plants grown in polluted soil. Decline percent in hydrocarbon at these treatment reached 75.3, 85.7, and 75.6%, respectively.
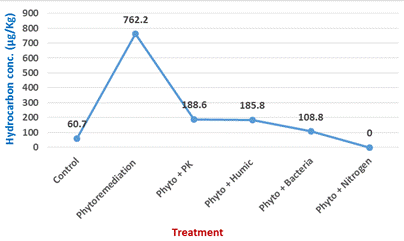
Figure 3. Hydrocarbon content in wheat grains under different treatments of polluted soil and their control.
3.6. Estimation of Soil Microbial Community in Different Treatments
The variation in soil microbial community content between control (un- polluted soil) and different treatments of polluted soil were indirect estimated through determine the number of viable cells via spectrophotometry analysis (Table 6).
Table 6. The variation in soil microbial community content between control and treatments of polluted soil as determined via spectrophotometry analysis.
| Treatment | Optical Density |
| Control | 1.2 |
| Phytoremediation | 0.8 |
| Phyto + PK | 1.5 |
| Phyto + Humic | 1.7 |
| Phyto + Bacteria | 2.3 |
| Phyto + Nitrogen | 1.1 |
As shown in table (6), the most enriched soil with microbial community/content comparing with control was as following: Phyto + Bacteria treatment, Phyto + Humic treatment, Phyto + PK treatment, and Phyto + Nitrogen treatment. While, the Phytoremediation treatment exhibited the lowest enriched soil with microbial community/content comparing with control.
3.7. Analysis of Variations in Microbial Community Using RAPD and ISSR Markers
Molecular markers analysis of six DNA samples represent control and five phyto-chemical treatments were performed by using 10 RAPD decamer primers and 5 ISSR primer in order to explore the effect of the different treatments on structure of soil microbial community comparing with control (Figure 4). The RAPD reactions produced 138 scorable total bands, out of which 113 found to be polymorphic. For ISSR, used primer yielded 56 total bands, out of which 51 bands were polymorphic.
A dendrogram based on UPGMA analysis of the fingerprints/amplicons obtained from both RAPD and ISSR markers was constructed (Figure 5). The dendrogram comprise two main clusters, the first cluster (The major) was subsequently divided into two subclusters; the first subcluster comprised two sub-subclusters. The first sub-subcluster including the Phytoremediation treatment and Phyto. + Humic acid treatment. Meanwhile, the second sub-subcluster including the control and Phyto. + PK treatment. While, the second subcluster comprised the Phyto. + Nitrogen treatment. Meanwhile, the second cluster involved only the Phyto. + Bacteria treatment.
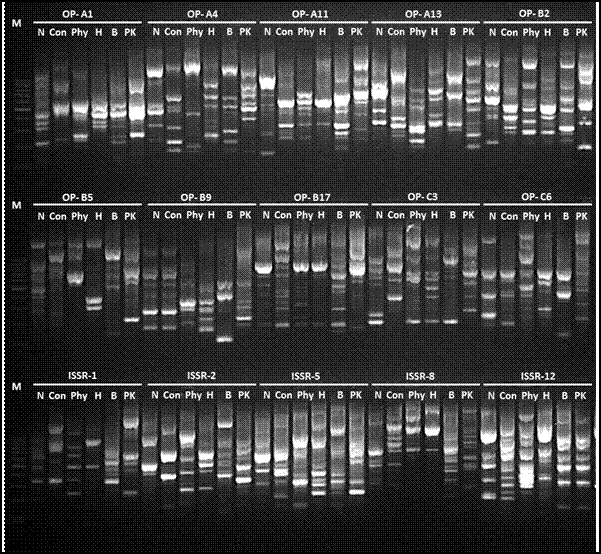
Figure 4. Agarose gel illustrate the RAPD and ISSR pattern variations of soil microbial communities content between control and treatments as determined via spectrophotometry analysis.
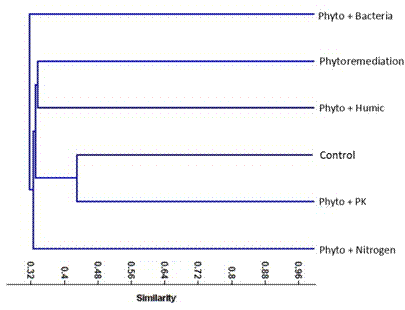
Figure 5. Phylogenetic analysis based on combined data obtained from ISSR and RAPD markers.
4. Discussion
4.1. Effect of Spent Engine Oil on Soil Properties
Oil pollution could lead to significant changes in soil physiochemical properties, such as bulk density, soil organic carbon and organic matter, holding capacity, moisture content and hydraulic conductivity, NH4, and NO3. These data are agreed with that of Kayode et al. [25] reported increased bulk density in soil contaminated with spent lubricant oil. The hydrophobic nature of PHCs influences the water holding capacity and moisture content of soils. Studies have shown that soils polluted with PHCs are characterized by lower water holding capacity, moisture content and hydraulic conductivity compared with unpolluted soils, also reduced soil pH together with increases in soil organic carbon and organic matter on crude oil polluted soils have been recorded [26]. Increases in total nitrogen, NH4, and NO3 have also been observed on these soils that polluted with PHCs these data agreement to Marinescu et al. [27]. The increase in percent organic carbon and Nitrogen of spent engine oil treated soil relative to control could be attributed to structural of spent engine oil that applied to soil. Okonokhua et al. [28] reported increase in carbon and nitrogen of spent oil treated soil relative to control. The highest values of P, K, Na, Ca and Mg were recorded in polluted soil compared to unpolluted soil. Also there were increases of heavy metals content in polluted soil sample than in unpolluted soil. Reduced soil pH caused by the presence of PHC in soils also favours the availability of heavy metals which may be absorbed by crops growing on this soil and this can be toxic to them [29]. These data may be attributed to the nature of the polluting substance as well as the initial soil properties [30]. Generally, soil that is polluted with spent engine oil as source of hydrocarbon (PHCs) is different from unpolluted soils. These change due to changes in their biological as well as physicochemical properties [31]. Oil pollution could lead to significant changes in soil chemical properties, such as TPH, TOC, C/N and C/P ratios [32].
4.2. Effects of Remediation Treatments on Hydrocarbon Degradation
In the present study, the treatments of Phyto-Chemical remediation enhanced the degradation of TPH significantly and obviously prolonged the validity of Phyto-Chemical compared with the Phyto separately. Hydrocarbon has been reported to bind to humic substances strongly depending on the aromaticity of the humic material [33]. Humic substances possess many functional groups and have good sorption characteristics. From the bioremediation point of view this usually leads to immobilization and consequent decrease in pollutant toxicity [34]. On the other hand, humic substances can increase bioavailability of pollutants for degrading microorganisms among other, by acting as surfactants [35]. In the presence of permanganate ions, chemical oxidation can occur [36]. In potassium permanganate oxidation, hydrocarbon which are in contact with the soil matrix components are oxidized and their concentration will decrease [37]. Permanganate ions quickly oxidize hydrocarbon alkene carbon-carbon double bonds [36]. Ferrarese et al. [38] showed that the oxidation reactions were frequently rapid and appear to be completed within few hours. However, in order to assess the total removal efficiency of different reactants including potassium permanganate, the reactions were not quenched and were allowed to continue until the complete consumption of all chemicals before being analysed. The resulting products of chemical oxidation may or may not be more biologically toxic than the original compound [39].
4.3. Effect of Spent Engine Oil on Growth and Chemical Composition of Wheat Plants
The results clearly showed that Spent Engine Oil contamination affects on the growth parameters of wheat plants. Results showed that there was significant difference in the chemical composition of the wheat grain grown in the polluted soil, and those grown in the unpolluted soil. This could be as a result of a hydrophobic layer over the roots forward by the spent engine oil, which may have limited water and nutrients absorption necessary for synthesis of protein and fat in plant. This observation is in line with the findings of Ogbuehi et al. [40] and Agbogidi et al. [41] who reported that reduction in protein, crude fiber and at contents of cassava and maize respectively was due to impairment of photosynthetic activities through cell injury and disruption of cell membrane caused by properties of crude oil. Also, carbohydrates increased as results of hydrocarbon treatments. These findings may be due to the effect of hydrocarbon pollutants on metabolism, mobilization and translocation of carbohydrates.
4.4. Effect of Spent Engine Oil Pollution on Macronutrients of Wheat Plant and Soil After Harvesting
Macronutrient contents of wheat grown in the unpolluted soil recorded the higher values than macronutrient contents of wheat plants grown in the spent oil polluted soils. These data agree with Agbogidi et al., [41] who reported that petroleum products are known to reduce nitrogen availability in the soil. This could be the cause of adverse effect on the plant growth parameters in diesel oil polluted soil. The effect of addition of nutrient amendment on diesel polluted soil was found to ameliorate the soil condition and enhanced the growth performance of plant. The adverse effects could be due to disruption of the absorption and uptake of nutrients by petroleum products of the polluted soil [42]. These nutrients (nitrogen, phosphorus, and potassium) are essential to plant growth and development hence reduction in their bioavailability will lead to reduction in plant growth. Similarly, reduction in some essential plant nutrients such as nitrogen and phosphorus in PHC-polluted soil [43] may affect proper crop development on these soils. PHCs alter the fertility status of soils and hence reduce their ability to support proper crop growth and development [44]. From the results, it can be concluded that HA, B and PK addition to Phyto are effective remediation materials for diesel oil polluted soil and at the same time restored the fertility of the soil, thus enhancing plant growth and timber productivity.
4.5. Content of Hydrocarbons by Wheat Grown Under Different Treatments
This study was investigated the content of TPH by wheat grown in different field-contaminated soils. Naturally, uptake of hydrocarbon plant increase as the concentration of hydrocarbon soil increase and translocation of hydrocarbon that depended on their chemical properties [45]. Results indicate that wheat plant was effective and promising for the removal of TPH from highly contaminated soil. Additives of organic and inorganic compound may promote plant growth even in oil contaminated soils and thereby positively affect phytoremediation efficiency. Moreover, the improvement of soil nutrient conditions through this addition can further enhance hydrocarbon biodegradation. Since the main mechanism of phytochemical in oil-polluted soils is based on the stimulation of soil micro-organisms, it can be assumed that the higher root biomass obtained with plants provides a larger rhizosphere for the microbial population and, therefore, an enhanced degradation of petroleum hydrocarbons in soils [46]. Tejada et al. [47] also observed that oil degradation could possibly be further enhanced by improving plant growth through fertilizer optimization.
4.6. Analysis of Variations in Microbial Community Using RAPD and ISSR Markers
Petroleum hydrocarbons in nature are degraded by diverse groups of soil microorganisms, which have capability to utilize hydrocarbons as a sole source of carbon and energy. Exploration and documentation of microbial diversity in a TPH-contaminated soil is crucial because it helps to identify novel bacterial strains capable of degrading a wide range of pollutants. Moreover, they give a background about bacterial diversity and community changes in various pollutant-degrading communities. Moreover, the exploration of the effect of different Phyto-chemical treatments on the soil microbial communities represent a key and initial step for developing any bioremediation strategy.
The obtained results from RAPD and ISSR marker systems successfully revealed a discriminative pattern between the DNA isolated from soil microbial communities of control and treatments. The cluster analysis results exhibited that the Phyto. + Bacteria treatment was clustered individually, this may be due to the directional enrichment of soil microflora with particular type of bacteria (Pseudomonas aeruginosa bacteria addition). In this context, La Rosa et al., [22] studied microbial diversity in a polycyclic aromatic hydrocarbon-impacted soil by 16S rRNA gene sequencing and amplified fragment length polymorphism (AFLP) analysis. They results showed that AFLP marker had the ability to differentiate and track related closely microbes is fundamental for studying structure and dynamics of microbial communities in contaminated ecosystems.
While, Patel and Behera [10] assessed the genetic diversity between 18 metagenomes of Coal mine spoil and their impact on the microbial ecosystem using twenty RAPD decamer primers. They results indicated that different coal mine spoils, through microbiologically distinct, are interlinked in a sequence as per the age series which reflect the enrichment of genetic diversity due to the reclamation progress with the age of coal mine spoil. Also, Tilwari et al., [11] investigated the microbial diversity of industrially contaminated and uncontaminated agriculture field soil using random amplified polymorphic DNA (RAPD) analysis. They results confirmed the effects of pollution on the distribution and biodiversity of soil microorganisms where most of the native beneficial microorganisms were disappeared or not cultured under these stress conditions as compared to the normal agricultural field soils, which is certainly affecting soil fertility and productivity.
Finally, the availability of simple molecular techniques such as (RAPD, ISSR, ….ect.) for fast and reliable genotypic characterization should increase our knowledge of ecology, structure and dynamics of microbial communities in contaminated ecosystems. Documentation of microbial diversity at petroleum-impacted sites will help to formulate novel strategies for efficient and effective reclamation of contaminated sites.
5. Conclusion
This study recommend avoiding the uses of phytoremediation approach separately for cleaning up hydrocarbon contaminated soils due to the high accumulation ratio of TPH in plant grains, which consequently can represent a toxic ratio for human and animal consumption. Therefore, approaches combining the phytoremediation with other organic and inorganic compound (such as humic acid, potassium permanganate and bacteria) were recommended due to their ability to degrade the TPH in contaminated soil without accumulate a higher ratio inside the wheat plant grain. Moreover, additives of organic and inorganic compounds to phytoremediation treatment represent a significant positive effect on the microbial communicates in contaminated soil.
References
- US EPA. (2000). Introduction to phytoremediation. Environmental Protection Agency, USA. Page 5.
- Huang XD, El-Alawi Y, Gurska J, Glick BR, Greenberg BM (2005). A multi-process phytoremediation system for decontamination of persistent total petroleum hydrocarbons (TPHs) from soils. Microchem. J. 81: 139-147.
- Khan AG (2005). Role of soil microbes in the rhizospheres of plants growing on trace metal contaminated soils in phytoremediation. J. Trace Elem. Med. Biol. 18: 355-364.
- Edwin-Wosu NL, Albert E (2010). Total Petroleum Hydrocarbon Content (TPH) As an Index Assessment of Macrophytic Remediation process of a Crude Oil Contaminated Soil Environment. J. Appl. Sci. Environ. Manage. March, 14 (1) 39–42.
- Kim D, Woo SM, Yim J, Kim T, Thao N, Ngoc P, Lee J, Kang L, Gwang H (2010). The feasibility of phytoremediation combined with bioethanol feedback production on diesel –contaminated soil. In: 19th World Congress of Soil Science, Soil Solutions for a Changing World. Brisbane Australia, 66-69.
- Whiteley AS, Bailey MJ (2000). Bacterial community structure and physiological state within an industrial phenol bioremediation system. Appl. Environ. Microbiol. 66, 2400–2407.
- Carvalho MF, Alves CT, Ferreira MM, De Marco P, Castro PL (2002). Isolation and initial characterization of a bacterial Consortium able to mineralize fluorobenzene. Appl. Environ. Microbiol. 68, 102–105.
- Kaplan CW, Kitts CL (2004). Bacterial succession in a petroleum land treatment unit. Appl. Environ. Microbiol. 70, 1777–1786.
- Schneegurt MA, Kulpa Jr CF (1998). The application of molecular techniques in environmental biotechnology for monitoring microbial systems. Biotechnol. Appl. Biochem 27, 73–79.
- Patel AK, Behera N (2011). Genetic diversity of coal mine spoil by metagenomes using random amplified polymorphic DNA (RAPD) marker. Indian J. of Biotechnology, 10, 90-96.
- Tilwari A, Chouhan D, Sharma R (2013). Random amplified polymorphic DNA (RAPD) analysis of microbial community diversity in soil affected by industrial pollutants: Reference to Mandideep industrial area. African J. of Microbiology Research, 7(30), 3933-3942.
- Sadoudi AD, Ali AS, Dahmani Y, Chergui R (2014). Treatment of oil polluted soil by injecting Pseudomonas aeruginosa and produced rhamnolipid. International Journal of Environmental Engineering Science and Technology Research, 2 (1): 1–9: 2326–3113.
- Page AL, Miller RH, Keeney DR (1982). Methods of Soil Analysis. II: Chemical and Microbiological Properties, 2nd ed. Am. Soc. Agron. Inc.; Soil. Soil Sci Soc. Am. Inc, Madison, Wisconsin U.S.A.
- IOC (Inter-govemmental Oceanographic Commission) (1984). Manuals and Guides No. 13: Procedures for the petroleum components of the IOC Marine Pollution Monitoring System (MARPOLMONP). Paris, UNESCO, 35p.
- Jackson ML (1973). Soil Chemical Analysis. Pentice Hall of India Pvt. Ltd., New Delhi.
- Lindsay L, Norvell WA (1978). Development of DTPA soil test for zinc, iron, manganese and copper. Soil Science Society American Journal, 42: 421-428.
- AOAC (1970). "Official Methods of Analysis". A. O. A. C., Washington, D. C.
- AOAC (1990). "Official Methods of Analysis". A. O. A. C., Washington, D. C.
- Smith D, Paulsen GM, Raguse CA (1964). Extraction of total available carbohydrates from grass and legume tissue. Plant Physiol. 39: 960 –962.
- Murphy RP (1958). Extraction of plant samples and the determination of total soluble carbohydrates. J. Sci. Food Agric. 9, 714-717.
- Snedecor GW and Cochran WG (1980). Statistical Method. 7th Ed., Iowa State Univ. Press, Ames, Iowa, USA.
- La Rosa G, De Carolis E, Sali M, Papacchini M, Riccardi C, Mansi A, Paba E, Alquati C, Bestetti G, Muscillo M (2006). Genetic diversity of bacterial strains isolated from soils, contaminated with polycyclic aromatic hydrocarbons, by 16S rRNA gene sequencing and amplified fragment length polymorphism fingerprinting. Microbiological Research, 161, 150—157.
- Adawy SS, Atia MAM (2014). A multidisciplinary molecular marker approaches to assess the genetic diversity in Egyptian date palm. Int. J. of Bio-Technology and Research, 4 (6), 1-12.
- Jaccard P (1908). Nouvelles rescherches sur la distribution florale. Bulletin Sociètè Vaudoise des Sciences Naturelles, 44: 223-270.
- Kayode J, Oyedeji A, Olowoyo O (2009). Evaluation of the effect of pollution with spent lubricant oil on the physical and chemical properties of soil. Pacific J. Sci. Tech. 10(1):387-391.
- Nwaoguikpe RN (2011). The effect of crude oil spill on the ascorbic acid content of some selected vegetable species: Spinacea oleraceae, Solanum melongena and Talinum triangulare in an oil polluted soil, Pakistan journal of nutrition, 10 (3): 274-281.
- Marinescu M, Toti M, Tanase V, Plopeanu G, Calciu I, Marinescu M (2011). The effects of crude oil pollution on physical and chemical characteristics of soil, Research Journal of Agricultural Science, 43(3): 125-129.
- Okonokhua B O, Ikhajiagbe B, Anoliefo G O, Emede J O (2007). The effect of spent engine oil on soil properties and growth of maize (Zea mays L.). J. Appl. Sci. Environ. Mgt 11(3):147-152.
- McBride MB (1994). Environmental Chemistry of Soils, Oxford University Press, New York.
- Semple KT, Morriss A W J, Paton G I (2003). Bioavailability of hydrophobic organic contaminants in soils: fundamental concepts and techniques for analysis, European journal of soil science, 54: 809-818.
- Robertson SJ, McGill WB, Massicotte HB, Rutherford PM (2007). Petroleum hydrocarbon contamination in boreal forest soils: A mycorrhizal ecosystems perspective, Biological Reviews, 82, 213-240.
- Wang X, Feng J, Zhao J (2010). Effects of crude oil residuals on soil chemical properties in oil sites, Momoge Wetland, China. Environ. Monit. Assess. 161: 271–280.
- Gauthier TD, Seitz WR, Grant CL (1987). E ects of structural and compositional variations of dissolved humic materials on pyrene K oc values. Environmental Science and Technology, 21: 243–248.
- Dercová K, Sejáková Z, Skokanová M, Barančíková G, Makovníková J (2007). Bioremediation of soil contaminated with pentachlorophenol (PCP) using humic acids bound on zeolite," Chemosphere, 66 (5), 783–790.
- Fava F, Berselli S, Conte P, Piccolo A, Marchetti L (2004). Effects of humic substances and soya lecithin on the aerobic bioremediation of a soil historically contaminated by Polycyclic Aromatic Hydrocarbons (PAHs), Biotechnology and Bioengineering, 88 (2): 214–223.
- Brown GS, Barton LL, Thomson BM (2003). Permanganate oxidation of sorbed polycyclic aromatic hydrocarbons. Waste Management, 23, 737-740.
- Silva SA, De PT, Silva DA, Barros Neto B, Simonnot MO (2009). Potassium permanganate oxidation of phenanthrene and pyrene in contaminated soils. Journal of Hazardous Materials. 168: 1269-1273.
- Ferrarese E, Andreottola G, Oprea IA (2008). Remediation of PAH-contaminated sediments by chemical oxidation. J. Hazard. Mater., 152 (1), 128-139.
- Dabestani R, Ivanov I (1999). A compilation of physical, spectroscopic and photophysical properties of poly aromatic hydrocarbons. Photochemistry and Photobiology, 70: 10-34.
- Ogbuehi HC, Ezeibekwe IO, Agbakwuru U (2010). Assessment of Crude Oil Pollution the proximate composition and macro element of cassava crop in Owerri, Imo State. Int Sci Res J (2): 62-65.
- Agbogidi OM, Erutor PG, Appanbi SO, Nagi GU (2007). Evaluation of crude oil contaminated soil on the mineral nutrient element of maize (Zea mays L.), J Agron. 6 (1): 188-193.
- Njoku KL (2008). Evaluation of Glycine max and Lycopersicon esculentum in the remediation of crude oil polluted soil. Ph.D. Thesis, Submitted to the School of Postgraduate Studies, University of Lagos, pp: 200.
- Akpoveta OV, Egharevba F, Medjor OW, Osaro KI, Enyemike ED (2011). Microbial degradation and its kinetics on crude oil polluted soil, Research journal of chemical sciences, 1 (6): 8-14.
- Abii TA, Nwosu PC (2009). The effect of oil-spillage on the soil of Eleme in Rivers State of the Niger Delta area of Nigeria, Research journal of environmental sciences, 3 (3): 316-320.
- Tao S, Jiao XC, Chen SH (2006). Accumulation and distribution of polycyclic aromatic hydrocarbons in rice (Oryza sativa). Environ Pollut, 140 (3): 406-415.
- Shirdam R, Ali DZ, Gholamreza NB, Nasser M (2008). Phytoremediation of hydrocarbon-contaminated soils with emphasis on the effect of petroleum hydrocarbons on the growth of plant species. Phytoprotection, 89: 21-29.
- Tejada M, Gonzalez JL, Hernandez MT, Garcia C (2008). Application of different organic amendments in a gasoline contaminated soil: Effect on soil microbial properties. Bioresour. Technol. 99: 2872-2880.

 (M. A. M. Atia)
(M. A. M. Atia)