Microalgae Community Composition in Tropical Temporary Freshwater Ponds of Burkina Faso (West Africa)
Bilasse Zongo1, 2,*, Frederic Zongo1
1Laboratory of Biology and Ecology of Plants, University of Ouagadougou, Ouagadougou, Burkina Faso
2Laboratory of Study and Research on Natural Resources and Environmental Sciences, Polytechnic University of Bobo-Dioulasso, Bobo-Dioulasso, Burkina Faso
Email address

(B. Zongo)
*Corresponding author
Citation
Bilasse Zongo, Frederic Zongo. Microalgae Community Composition in Tropical Temporary Freshwater Ponds of Burkina Faso (West Africa). American Journal of Agricultural Science. Vol. 3, No. 5, 2016, pp. 72-84.
Abstract
Microalgae remain an important component in aquatic ecosystems and play a basic role in the functioning of these ecosystems. Factors controlling the composition of the species types, distribution and biomass of microalgae are physicochemical and biological parameters and hydrologic cycle strongly affected by the climatic conditions. Therefore, microalgae could be used to characterize water quality due to their strong sensitivity to the change in water variables. Investigations in 86 temporary ponds of the sahelian and sudanian zones of Burkina Faso which water is still used by local population have been conducted in 2007, 2008, 2009. The study planned to document the community composition of algae in temporary ponds. From those investigations, 291 species belonging to eucaryotic phylums (streptophyta, euglenophyta, bacillariophyta and dinophyta) and cyanobacteria have been recorded. The most represented phylum was streptophyla, making the desmidiaceae the most represented family. The variation in environmental conditions allowed strong variation in species composition and occurrence. Low number of species was frequent in ponds but major of them is cosmopolitan. Nevertheless, comparing recorded species with the data on species previously recorded in Burkina Faso reveals that, 151 (52% of recorded species) species were identified for the first time in the country. Temporary ponds provide good conditions for algae growth in the presence of water as they accumulate compounds from runoffs polluting water. A use of temporary pond’s water by local populations is not safe for a healthy life. Therefore measures have to be taken to avoid clean water scarcity and consumption of temporary pond water by populations.
Keywords
1. Introduction
Microalgae remain an important component in all water systems and play a basic role in the functioning of aquatic ecosystems. Within aquatic ecosystems, factors controlling the composition of the species types, distribution and biomass of microalgae are physicochemical and biological parameters as well as hydrologic cycle [1-3]. These factors are also strongly affected by the climatic conditions. Therefore, the type and the increasing level of microalgae particularly phytoplankton can vary from an ecosystem to another because of the variation in environmental variables. The latter could also vary while going from a habitat to another and a period to another [4]. Therefore, in certain conditions, we assist to an excessive increase of microalgae biomass due to eutrophication that leads to an absence of light and dissolved oxygen in water. However, in other aquatic systems conditions with very lower organic matter and nutrient, negligible algae biomass is produced. Because of their higher sensitivity to the water quality changes and their ability to characterize the trophic level of water systems, numerous algal communities are used to biologically determine water quality level in aquatic ecosystems [5-6].
Temporary ponds seem to be the most exposed ecosystems to climatic conditions and anthropogenic factors as they have very small sizes and are not long-lasting. So, temporary ponds parameters do highly vary with time and space. Provided in water, only by precipitations, water level in temporary ponds decreases quickly and can totally finish in sahelian and sub-sahelian areas due to rainfall irregularity. However, these ecosystems are sometimes omitted in the field of research while in sub-saharan regions of Africa they still remain important for populations and their cattle using unclean water for their own consumption [1, 7]. Zongo et al. [7] in a previous study focused their investigations on water quality of temporary ponds in forests and villages and demonstrated the impact of human activities on temporary ponds water quality degradation, and the role of forests to protect those ponds and the quality of water contained herein. Microalgae species composition is an important tool for water quality monitoring because many species are used as indicators of the ecological conditions and water quality of their ecosystems [8]. Therefore, the present research aims to document microalgae community composition in temporary freshwater ponds in order to provide more scientific and ecological data from the tropical water habitats but also to give a better knowledge on temporary pond water quality, which water is used by local populations in the villages. Specifically, it aims at measuring the species richness of microalgae in ponds, determining the occurrence of those species and classifying them according to their geographical repartition.
2. Material and Methods
2.1. Study Sites
Investigations have been conducted in the central, eastern regions (sudanian zone) and northern region (sahelian zone) of Burkina Faso (West Africa). The study area lies between the latitudes 12 75’ and 16 50’ North and the longitudes 06 00’ and 09 75’ East (Fig. 1). The area is a tropical zone characterized by two seasons: a rainy season from June to September (mean rainfall: 709 to 786 mm and 400 to 600 mm per year respectively in the sudanian and the sahelian zones) with a maximum rainfall in August and a dry season from October to May. March, April and May are the warmest months of the year and the temperature can rich 40 to 45°C while December and January are the coldest ones with an average of about 20°C.
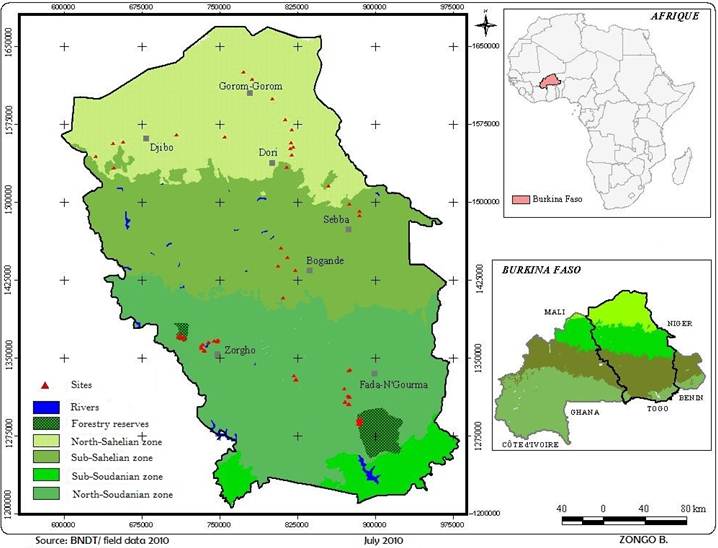
Figure 1. Study area and sampling sites location.
2.2. Ponds Sample Design
One month before starting the investigations in the field, the whole study area was explored for pond geo-localization, inventory and selection. Therefore, during explorations, approximately 100 temporary ponds were recorded; however in the case of ponds located close to each other (200 m or less distance between them), only one was randomly chosen for the investigations.
Hence, eighty six temporary ponds have been randomly selected for micro-algae samples collection. In the sudanian zone sixty-one ponds (30 in the central zone and 31 in the eastern region) were selected. In this part of the study area, investigations have been conducted including ponds belonging to protected areas (reserve of Wayen and reserve of Pama respectively in the central and eastern regions). In the central part, 15 temporary ponds in the reserve of Wayen and 15 temporary ponds in the villages and fields have been investigated. In the eastern part, 15 temporary ponds have been selected in the reserve of Pama and 16 outside the reserve (villages and fields). However, in the northern sahelian part of the country, 25 ponds widespread in the whole area have been visited for data collection.
2.3. Collection of Samples and Species Identification
Algae samples collection was done twice a year (in August and September) during three years (2007, 2008 and 2009). Collections were realized in the open water using a tube of 120 ml following the procedure implemented by Zongo [9]. Sampling tubes were transferred into collection bottles and immediately preserved with 5% formalin at ambient temperature. Species of algae were identified following species identification procedure as described by Zongo et al. [10] and using the taxonomical criterias of algaebase [11].
2.4. Community Composition Study
A list of identified species per site was realized. Species richness of site and the frequency of occurrence [12] were determined using the list. According to their occurrence, the species have been classified as following:
• Frequent species: recorded in at least 50% of temporary ponds,
• Less frequent species: present in 25% to 49% of temporary ponds,
• Rare species: recorded in less than 25% of temporary ponds.
Furthermore, species were classified in taxonomic phylums and their spatial repartition also determined [13]. The different phylums (cyanoprocaryota, streptophyta, euglenophyta, bacillariophyta and dinophyta) were obtained according to the taxonomic groups of planktonic algae occurring in freshwater habitats [14, 15, 16].
The spatial distribution of species was done following the chorological groups established by White [17] and using literature [e.g., 18]. Therefore, the following groups were taken into account:
• Cosmopolitan (Cosm.): species occurring in all regions of the world,
• Subcosmopolitan (Subcom.): species occurring in all tropical regions,
• Paleotropical (Pal.): species in tropical Africa areas as well as in the tropical areas of Asia, Australia and Madagascar,
• Pantropical (Pan.): species occurring in the whole tropical regions of the world
• Afro-American (AA): species occurring in Africa and in the tropical America,
• Afro-Tropical (AT): species distributed only in tropical areas of Africa.
3. Results
3.1. Species Composition in Ponds
The microscopic observations of collected samples from temporary ponds allowed recording 291 species. Among them, 252 were found in the ponds of the sudanian area and 162 in those of the sahelian zone (fig. 2). They belong to 65 genera, 24 families and 5 phylums (table 1).
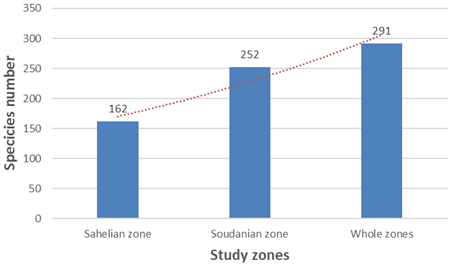
Figure 2. Species number in study zones.
Streptophyta is the most represented phylum with 57% of the total recorded species (Fig. 3). This group is followed by euglenophyta (23% of the species), cyanobacteria (12% of the species), bacillariophyta (7% of the species) and dinophyta (1% of the species).
The total number of procaryotic species was 34 (12%) belonging to two families of cyanobacteria phylum: the hormogonaceae composed by filamentous species and the chroococcaceae composed by mucilaginous species. However, the most represented species were eukaryotic with a total number of 257 representing 88% of the total recorded species.
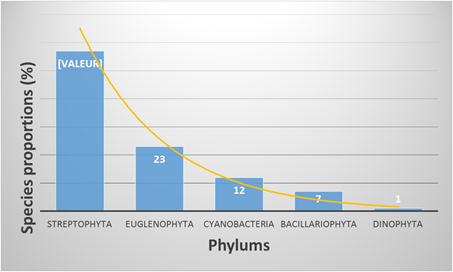
Figure 3. Species number in the different phyllums.
The following families were mostly represented in ponds: desmidiaceae with the highest number of species (89 species), euglenaceae (68 species), closteriaceae (31 species) and oscillatoriaceae (25 species). They are followed by the ones represented by lower number species less than 25 but more than 4 species each: zygnemaceae, chroococcaceae, scenedesmaceae, volvocaceae, bacillariaceae, pinnulariaceae, stauroneidaceae, selanastraceae, oocystaceae, coelastraceae (fig. 4). Ten other families represented together by only 18 species and composed by up to two species each were also recorded in temporary ponds in the sudanian as well as in the sahelian zones during the study period. They are: rivulariaceae, nostocaceae, chlorellaceae, dictyosphaeriaceae, oedogoniaceae, gonatogygaceae, peniaceae, amphipleuraceae, eunotiaceae, melosiraceae.
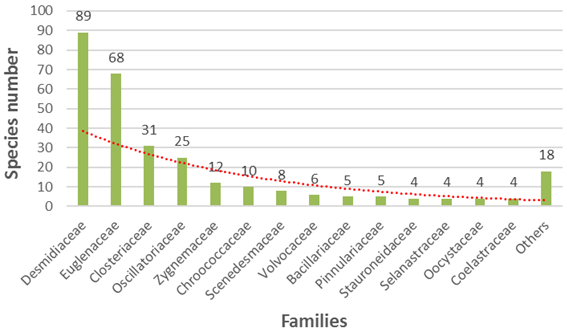
Figure 4. Species number in the different algae families.
3.2. Occurrence of Species in Ponds
There was a great variability in the species occurrence in the sampling sites. Among the species, 85-93% were characterized as rare species in ponds as they were recorded in less than 25% of the sampling sites (fig. 5, table 1). The less frequent species represented 6 to 13% respectively in the sudanian and sahelian zones. The number of frequent species was too low and estimated to 1% and 2% of the total recorded species in the sudanian and the sahelian zones. The frequent species are: Euglena proxima, Navicula sp., Pinnularia divergens, Trachelomonas raciborskii, Trachelomonas volvocinopsis, and Stauroneis sp. recorded in respectively 69%, 56%, 53%, 53%, 50% and 53% of sampling sites in the sudanian area. In the sahelian zone, Lepocinclis acus, Euglena proxima and Lyngbya martensiana were the frequent species and have been recorded respectively in 56%, 52% and 52% of sampling sites.
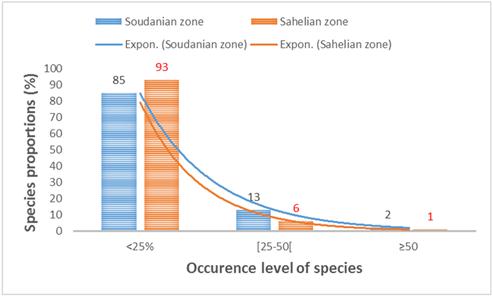
Figure 5. Occurrence of species in sampling sites.
Considering frequency occurrence of species in the sampling sites (fig. 6), Euglena proxima from euglenaceae family was the only species to be frequent in the sudanian zone as well as in the sahelian zone of Burkina Faso. Furthermore, euglenaceae was the most frequent family with 4 of the 8 most frequent species in the study area.
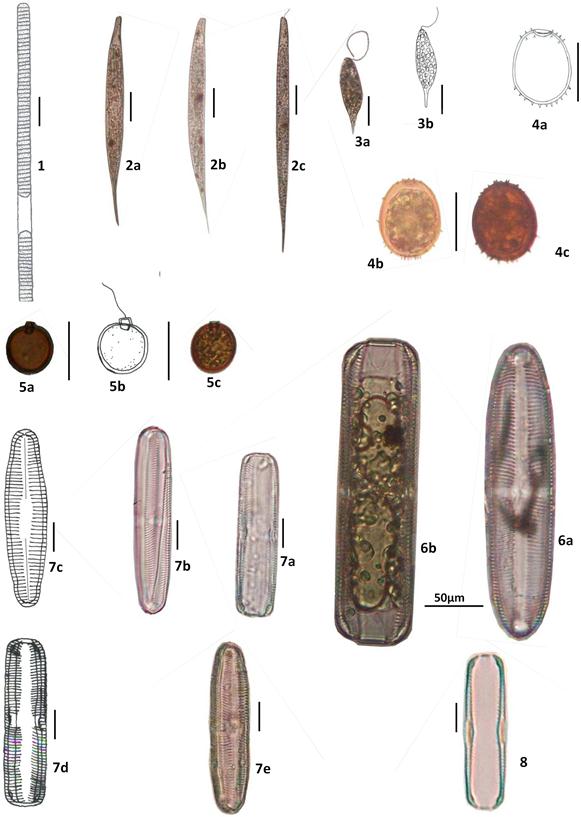
Figure 6. Pictures of frequent species in temporary ponds.
Note: 1: Lyngbya martensiana, 2: Lepocinclis acus, 3: Euglena proxima, 4: Trachelomonas raciborskii, 5: Trachelomonas volvocinopsis, 6: Navicula sp., 7: Pinnularia divergens, 8: Stauroneis sp. Non precised scale bars correspond to 25 µm
3.3. Geographical Distribution of Species
Among the different species, 42% were recorded in ponds of the sudanian zone as well as ponds of sahelian zone. However, considering their distribution in the world, the figure 6 shows that cosmopolitan species (59%) and subcosmopolitan (25%) were mostly recorded in temporary ponds (59%). They are followed by afro-tropical species (7%), paleotropical species (4%), pantropical (3%), and afro-american species (2%).
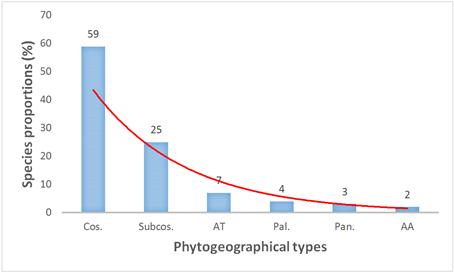
Figure 7. Geographical distribution of pond species in the world.
Note: Cos.: Cosmopolitan, Subcos.: Subcosmopolitan, AT: Afro-Tropical, Pal.: Paleotropical, Pan.: Pantropical, AA: Afro-American
4. Discussion
This study has led to bring to the forefront 151 species that have never been reported in Burkina Faso flora (table 1) according to precedent studies in the country [19]. They are belonging to the following 20 families: chroococcaceae, oscillatoriaceae, volvocaceae, chlorellaceae, oocystaceae, scenedesmaceae, selenastraceae, oedogoniaceae, gonatogygaceae, closteriaceae, desmidiaceae, euglenaceae, amphipleuraceae, bacillariaceae, pinnulariaceae, stauroneidaceae.
The species of streptophyta phylum were the dominated species of algae flora in temporary ponds. They are found to be dominant in freshwater ecosystems of West-Africa as reported by several studies [e.g., 20-23, 13]. In this study, streptophyta was particularly represented by desmidiaceae family that had the highest number of species in comparison to the other families of the phylum as well as families of the other phylums. Indeed, desmidiaceae represents a high species collection in almost the whole freshwater systems [24]. With 68% of streptophyta species and 36% of total algae flora recorded in ponds, this family highly contributes to the dominance of streptophyta in temporary ponds as well as freshwater habitats all over the world. Furthermore, according to Itlis [25], algae flora of the sudano-sahelian regions is highly dominated by streptophyta, but also euglenophyta, bacillariophyta and cyanobacteria while dinophyta (dinoflagellates) and rhodophyta are absent or represented by a low number of species. Dinophyta are minor component occurring in lakes and ponds [24] but a high species number of the phylum occurs in seas and oceans [26]. Therefore, only 03 species of dinoflagellate were recorded in tropical temporary ponds of Burkina Faso. Rhodophyta species were not recorded in ponds during investigations probably because most of them can only be developing and occurring in marine area [15]. Hence, no phylum occurring rather only in freshwater systems nor in marine ones. Nevertheless some of them (e.g. streptophyta and cyanobacteria), can be abundant within freshwater habitats [27]. Cyanobacteria species especially have ecological plasticity allowing them to colonize different kinds of water habitats [28] where they play an important role. Some species (e.g. Anabaena) are able to metabolize atmospheric nitrogen. This capacity is eased in certain filamentous algae by specialized cells named heterocytes [29]. Nitrogen fixation by cyanophyceae species can enrich oligotrophic habitats. However, they can particularly be source of blooms during eutrophication and contribute to the degradation of water habitats.
Euglenaceae, the only family of euglenophyta has the particularity to be composed by species with high occurrence in temporary ponds. It is composed by species characterizing by backwater rich in nutrients and organic matter and always associated to water sediments [24, 9]. Indeed, during rainy season, temporary ponds are enriched in nutrients and organic matter by runoffs. Those runoffs are the ones bringing residues and organic matters, justifying the recorded number of euglenaceae in ponds. Furthermore, most frequent species were euglenophyta (euglenaceae). This high frequency is certainly due to the fact that species of this phylum are able to encyst during negative conditions [30]. They are more able to adapt to the ecological conditions of habitats than the other species. However, bacillariophyta species occur in all freshwater habitats including backwater as well as flow water and can be used to characterize ecological changing of water [5-6, 24].
Because of strong change in climatic conditions during rainy season in the sudanian and the sahelian zones, great variation in species composition is observed in temporary ponds. The high variation in species composition during the period is characterized by the high number of non-frequent species and the low number of frequent species. Nevertheless, many species are cosmopolitan and found to be occurring in water habitats worldwide. Most algae species are able to grow in different conditions. They are widespread in aquatic habitats but can also occur in terrestrial and even space habitats of all continents [31]. Naturally, algae occur in wetlands as plankton, benthic and periphytic depending in the levels of salinity, organic matter, nutrient and the environmental temperature. Most species could occur in freshwater as well as marine habitats, tropical as well as temperate habitats [32]. Therefore, species occurring only in tropical conditions (Africa, America and Asia) represented by pantropical and paleotropical species with low number were recorded in temporary ponds in Burkina Faso.
5. Conclusion
Investigations run on the microalgae community composition of temporary ponds in Burkina Faso allowed to document the species diversity in sudanian and sahelian zones of Burkina Faso. Similar species to those occurring in freshwater habitats around the world have been recorded. Hence, an important portion of them is cosmopolitan while low number is confined to certain regions specifically, tropical regions of the world. This study pioneers in recording a lot of species that have been identified for the first time in Burkina Faso’s flora. It therefore contributes to a better knowledge of the microalgae species of the country but also of Africa and the whole world.
The variation of climatic conditions (e.g. irregular rainfall) plays an important role in the species composition in ponds. This has induced a strong temporal variation in the species composition with sometimes rarity of species. In the variation of species composition and diversity, the diversity of taxonomic groups, the occurrence of species in ponds within the study area, environmental variables influenced by climatic conditions play important role that needs to be investigated by further studies. The existence of some frequent species is due to their great ability to adapt to non-favorable conditions during lack of water in ponds. However, algae have been found to have a good and easy growth in temporary ponds in the presence of water because of accumulation of nutrient and organic matter from runoff. Indeed, water from temporary ponds used by local populations become a general health issue. Consequently, measures have to be taken to avoid consumption of temporary pond water by providing clean water to the populations in villages.
Acknowledgements
The authors would like to particularly thank Mark-Oliver Rödel, Adjima Thiombiano for their support. They are thankful to Meike Mohneke, Thiombiano Ahandi and Kaboré Jérémy for their help during the field work.
Appendix
Table 1. Occurrence of recorded species in temporary ponds.
Classes and
Families | Species | Sahelian zone | Soudanian zone |
Presence /
Absence | Occurence level | Presence /
Absence | Occurence level |
| Cyanophyceae | *Aphanocapsa conferta (W. West & G. S. West) Komárk.-
Lagn. & Cronb. | + | R | - | NR |
| Chroococcaceae | *Aphanocapsa koordersii Strøm | + | R | - | NR |
| Coelosphaerium kutzingianum Näg. | - | NR | + | R |
| *Coelosphaerium aerugineum Lemm. | - | NR | + | R |
| *Chroococcus goetzei Schmidle | + | R | - | NR |
| Chroococcus dispersus (Kleissler) Lemm. | + | R | + | R |
| *Chroococcus sp. | + | R | - | NR |
| *Merismopedia sp. | - | NR | + | R |
| Microcystis flos-aquae (Wittrock) Kirch. | - | NR | + | R |
| *Snowella litoralis (Häyrén) Kom. & Hind. | + | R | - | NR |
| *Woronichinia delicatula (Skuja) Kom. & Hind. | + | R | - | NR |
| Cyanophyceae | Lyngbya aghardii (Crouan) Gom. | + | R | + | R |
| Oscillatoriaceae | *Lyngbya bourrellyana Comp. | + | R | - | NR |
| *Lyngbya martensiana Meneghini ex Gom. | + | F | + | R |
| *Lyngbya major Gom. | + | R | + | R |
| *Oscillatoria boryana Gom. | - | NR | + | R |
| *Oscillatoria chalybea Gom. var. insularis Gardn. | - | NR | + | R |
| Oscillatoria limnetica Lemm. | - | NR | + | R |
| Oscillatoria limosa Gom. | + | R | + | R |
| | Oscillatoria princeps Vauch. ex Gom. | + | R | + | R |
| *Oscillatoria sancta (Kütz.) Gom. | + | LF | + | R |
| *Oscillatotia subbrevis Schm. | + | R | + | R |
| *Phormidium aerugineo-caeruleum (Gom.) Anag. & Kom. | - | NR | + | R |
| *Phormidium breve (Kützing ex Gom.) Anag. & Kom. | + | R | + | R |
| *Phormidium gietleri (Kisselev) Anag. & Kom. | - | NR | + | R |
| Phormidium hamelii (Frémy) Anag. & Kom. | - | NR | + | R |
| *Phormidium autumnale (Ag.) Gom. | + | R | - | NR |
| *Phormidium rimosum (Kom.) Anag. & Kom. | + | R | + | R |
| *Phormidium retzii Gom. | + | R | + | R |
| *Phormidium splendidum (Grev. ex Gom.) Anagn. & Kom. | - | NR | + | R |
| *Phormidium subfuscum Kütz. ex Gom. | - | NR | + | R |
| *Planctothrix mougoetii (Bory ex Gom.) Anagn. & Kom. | + | R | - | NR |
| *Planktothrix planctonica (Elinki) Anag. & Kom. | - | NR | + | R |
| *Pseudonabaena constricta (Sezafer) Lauterb. | + | LF | + | R |
| *Pseudonabaena catenata Lauterb. | + | R | + | R |
| Pseudonabaena limnetica (Lemm.) Kom. | + | LF | + | R |
| Chlorophyceae | Eudorina elegans Chodat | + | R | + | LF |
| Volvocaceae | *Eudorina californica Shaw | + | R | + | R |
| Gonium pectorale Müller | - | NR | + | R |
| Pandorina morum (Müller) Bory | + | LF | + | LF |
| *Stephanoon askenasii Schewiakoff | - | NR | + | LF |
| *Volvox aureus Ehr. | + | R | - | NR |
| Chlorophyceae | *Tetraedron hastatum (Reinsch) Chodat | + | R | + | R |
| Chlorellaceae | Tetraedron triangulare Korshikov | - | NR | + | R |
| Chlorophyceae | Actinastrum aciculare Playf. fo africanum (Huber-Pestalozzi) Comp. | + | R | + | R |
| Coelastraceae | Actinastrum hantzschii Largerheim | - | NR | + | R |
| Coelastrum microporum Näg. | - | NR | + | R |
| Chlorophyceae | Nephrocytium agardhianum Näg. | + | R | + | R |
| Oocystaceae | *Nephrocytium limneticum (G. M. Smith) Skuja | - | NR | + | R |
| Glaucocystis sp. | + | R | + | R |
Chlorophyceae
Scenedesmaceae | Crucigeniella crucifera (Wolle) Komárek | - | NR | + | R |
Pediastrum duplex Meyen var. gracillimum West et G.
S. West | + | R | - | NR |
| *Scenedesmus acutiformis Schröder | - | NR | + | R |
| Scenedesmus bicaudatus Dedusenko | + | R | + | R |
| *Scenedesmus crassus Chodat | - | NR | + | R |
| Scenedesmus disciformis (Chodat) Fott & Kom. | + | R | - | NR |
| Scenedesmus ecornis (Ehrenberg) Chodat | + | R | + | R |
| Scenedesmus obtusus Mayen | + | R | + | R |
| Scenedesmus quadricauda Chodat | + | R | + | R |
| Chlorophyceae | Ankistrodesmus fusiformis Corda ex Korshikov | + | R | + | R |
| Selanastraceae | Ankistrodesmus falcatus (Corda) Ralfs var. acicularis A. Br. Skuja | - | NR | + | R |
| *Ankistrodesmus convolutus Corda | + | R | + | R |
| Kirchneriella lunaris (Kirch.) Moeb. | + | R | + | R |
| Chlorophyceae | Oedogonium sp. | - | R | + | R |
| Oedogoniaceae | *Oedogonium tapeinosporum Wittr. | + | R | - | NR |
| *Oedogonium franconium O. Bock & W. Bock | - | NR | + | R |
| Zygnemaphyceae | Zygnema sp. 1 | - | NR | + | R |
| Zygnemaceae | Zygnema sp. 2 | + | R | - | NR |
| Zygogonium sp. | + | R | - | NR |
| Mougoetia sp. 1 | + | R | - | NR |
| Mougoetia sp. 2 | + | LF | + | R |
| Mougoetia sp. 3 | - | NR | + | R |
| Spirogyra sp. 1 | - | NR | + | R |
| Spirogyra sp. 2 | + | R | - | NR |
| Spirogyra sp. 3 | - | NR | + | R |
| Spirogyra sp. 4 | - | NR | + | R |
| Spirogyra sp. 5 | - | NR | + | R |
| *Spirogyra rhizobrachialis Jao | - | NR | + | R |
| Zygnemaphyceae | Gonatozygon aculeatum Hastings | + | R | + | R |
| Gonatogygaceae | Gonatozygon pilosum Wolle | + | R | + | R |
| *Gonatogygon kinahanii (Arch.) Ragh. | - | NR | + | R |
| Zygnemaphyceae | Penium margaritaceum Brébisson | - | NR | + | R |
| Peniaceae |
| Zygnemaphyceae | Closterium acerosum (Schrank) Ehrenberg | + | R | + | LF |
| Closteriaceae | *Closterium acutum Brébisson ex Ralfs var. latius
Grönblad | + | R | + | R |
| Closterium acutum Brébisson ex Ralfs var. variabile
(Lemm.) Kreiger | - | NR | + | R |
| *Closterium calosporum Wittr. var. brasiliense Börgessen | - | NR | + | R |
| *Closterium calosporum Wittr. var. majus West & G. S.
West | + | LF | + | LF |
| Closterium cornu Ralfs | - | NR | + | R |
| *Closterium costatum Corda ex Ralfs | + | R | + | R |
| *Closterium cynthia De Notaris | + | R | + | R |
| Closterium ehrenbergii Meneghini | - | NR | + | R |
| *Closterium ehrenbergii Meneghini var. atumidum
Grönblad | + | R | + | R |
| *Closterium ehrenbergii Meneghini var. malinvernianum
(De Not.) Rabenhorst | - | NR | + | R |
| *Closterium gracile Brébisson | + | R | + | R |
| *Closterium gracile Brébisson var. elongatum West & G. S. West | + | R | - | NR |
| *Closterium lanceolatum Kützing | - | R | + | R |
| Closterium leibleinii Kützing | + | R | + | R |
| *Closterium lineatum Ehrenberg ex Ralfs | + | R | + | R |
| *Closterium littorale Gay var. crassum West & West | - | NR | + | R |
| *Closterium macilentum Brébisson | - | NR | + | R |
| *Closterium malmei Borge | + | R | + | R |
| *Costerium malmei Borge var. semicirculare Borge | - | NR | + | R |
| Closterium nordstedtii Gutwinski | + | R | - | NR |
| Closterium praelongum Brébisson | - | NR | + | R |
| Closterium praelongum Brébisson var. brevius Nordsted | - | NR | + | R |
| Closterium pseudolunula Borge | - | NR | + | R |
| Closterium ralfsii Brébisson ex Ralfs var. hybridum
Rabenhorst | + | R | + | R |
| *Closterium strigosum de Brébisson | + | LF | + | R |
| *Closterium striolatum Ehrenberg | + | R | + | R |
| *Closterium striolatum Ehrenberg var. erectum Klebs | + | R | - | NR |
| *Closterium subfusiforme Messikommer | - | NR | + | R |
| Closterium tumidulum Gay | - | NR | + | R |
| *Closterium venus Kützing | + | R | + | LF |
| Zygnemaphyceae | *Actinattaenium cucurbitinum (Biss.) Teil. | + | R | + | R |
| Desmidiaceae | *Actinataenium australe (Raciborski) Croasdale | + | R | - | NR |
| Cosmarium aculeatum Forster var. africanum Couté et
Rousselin | - | NR | + | R |
| Cosmarium binum Nordsted | + | R | + | LF |
| *Cosmarium bioculatum Brébisson ex Ralfs | - | NR | + | R |
| Cosmarium connatum Brébisson ex Ralfs | + | R | - | NR |
Cosmarium contractum Kirch. var. ellipsoideum (Elfv.)
West & G. S. West | + | R | + | R |
| Cosmarium contractum Kirch. var. munitum (Delp.) West & G. S. West | - | NR | + | R |
| *Cosmarium creperum W. West & G. S. West. | - | NR | + | R |
| *Cosmarium crenatiforme Grönbl. | - | NR | + | R |
| Cosmarium decoratum W. West & S. G. West | + | R | + | R |
| *Cosmarium pseudodecoratum Schm. | - | NR | + | LF |
| *Cosmarium galeritum Nordst. | - | NR | + | R |
| *Cosmarium geminatum Lund. | - | NR | + | R |
| Cosmarium granatum Bréb. ex Ralfs | - | NR | + | R |
| *Cosmarium infirmum Grönbl. var. minus Grönbl. | + | R | - | NR |
| | *Cosmarium margaritatum (Lundell) Roy & Bisset var.
quadrum Krieger | + | R | + | R |
| | *Cosmarium monomazum Lundell var. dimaziforme
Grönbl. | + | R | + | R |
| | Cosmarium quasillus Lundell var. devorense Croasdale | - | NR | + | R |
| | *Cosmarium portianum Arch. | + | R | - | NR |
| | *Cosmarium wollei (West & G. S. West) Grönblad | + | R | + | R |
| | *Cosmarium subglobosum Nordstedt | - | NR | + | R |
| | Cosmarium pseudobroomei Wolle | + | R | - | NR |
| | *Cosmarium pyramidatum Bréb. ex Ralfs | - | NR | + | R |
| | *Cosmarium regulare Schmidle | + | R | + | R |
| | Cosmarium reniforme (Ralfs) Arcger | + | R | + | R |
| | *Cosmarium retusiforme var. africanum (Fritsch) Comp. | + | R | + | R |
| | Cosmarium stappersii Evens | - | NR | + | R |
| | Cosmarium subauriculatum W. West & G. S. West | + | R | + | LF |
| Cosmarium subauriculatum W. West & G. S. West var. bogoriense (Bern.) Bourr. | + | R | + | R |
| | *Cosmarium subcostatum Nordst. | - | NR | + | R |
| | *Cosmarium subdepressum W. West & G. S. West | + | R | - | NR |
| | *Cosmarium subhammeri Rich var. africanum Bourr. | + | R | - | NR |
| Cosmarium subspeciosum Nordst. | - | NR | + | R |
| *Cosmarium subreniforme Nordst. | - | NR | + | R |
| *Cosmarium subtumidum Nordst. | - | NR | + | R |
| *Cosmarium subundulatum Wille | - | NR | + | R |
| | *Cosmarium tetragonum (Näg.) Archer. | - | NR | + | R |
| | *Cosmarium turpinii Bréb. var. intermedium Krieg. | + | R | + | R |
| | Euastridium verrucosum Carter | + | R | - | NR |
| | *Euastrum denticulatum (Kirch.) Gay | + | R | + | R |
| | *Euastrum didelta Ralfs | - | NR | + | R |
| | *Euastrum dubium Näg. | + | R | - | NR |
| | Euastrum dubium Näg. var. latum Krieg. | + | R | - | NR |
| | *Euastrum germanicum (Schm.) Krieg. | + | R | + | R |
| | *Euastrum subhypochondrum Fritsch et Rich var. eximum (Grönbl. et Scott) Bourr. et Couté | + | R | - | NR |
| | Euastrum subhypochondrum Fritsch & Rich var.
croasdaleae Comp. | + | R | + | R |
| | Euastrum sp. | + | R | + | R |
| | Euastrum spinulosum Delp. | - | NR | + | R |
| | Euastrum spinulosum Delp. var. lindae Grönbl. & Scott | - | NR | + | R |
| | Hyalotheca dissiliens Bréb. ex Ralfs | - | NR | + | R |
| | *Hyalotheca dissiliens Bréb. ex Ralfs fo. bidentula Nordst. | + | R | - | NR |
| | *Hyalotheca ondulata Nordst. | - | NR | + | R |
| *Micrasterias americana Ralfs var. hybrida Woo. & tweed | + | R | + | R |
| | *Micrasterias apiculata (Ehr.) Menegh. | - | NR | + | R |
| | *Micrasterias conferta Lund. | - | NR | + | R |
| | *Micrasterias mahabuleswarensis Hobs. var. comperei
Couté et Rousselin | - | NR | + | R |
| | *Micrasterias moebi Borge var. ridleyi West & G. S. West | - | NR | + | R |
| | Micrasterias sp. | - | NR | + | R |
| | *Micrasterias radians Turn. var. evoluta (Turn.) Krieger | - | NR | + | R |
| | *Micrasterias thomassiana Arch. var. notata (Turn.)
Krieger | - | NR | + | R |
| | *Pleurotaenium caldense Nordst. | + | R | - | NR |
| | Pleurotaenium ehrenbergii (Ralfs) De Bary | + | R | + | LF |
| | Pleurotaenium ovatum Nordst. | + | R | + | R |
| | Pleurotaenium subcoronulatum (Turn.) West. & G. S. West | + | R | + | R |
| | Pleurotaenium trabecula (Ehr.) Näg. | - | NR | + | R |
| | Staurastrum bineanum Rabh. | - | NR | + | R |
| | *Staurstrum muticum Ralfs | + | R | + | R |
| | Staurastrum setigerum Cleve | + | R | + | R |
| | Staurastrum setigerum Cleve var. occidentale West. & G. S. West | + | R | - | NR |
| | *Staurastrum hirsutum (Ehr.) Bréb. ex Ralfs | + | R | - | NR |
| *Staurastrum hystrix Ralfs | + | R | + | R |
| *Staurastrum leptacanthum Nordst. var. borgei Förster | + | R | - | NR |
| | *Staurstrum manfedtii Delp. | + | R | + | R |
| | *Staurastrum polymorphum Bréb. ex Ralfs | - | NR | + | R |
| | Staurastrum sp. 1 | - | NR | + | R |
| | Staurastrum sp. 2 | - | NR | + | R |
| *Staurastrum tohepekaligense Wolle | - | NR | + | R |
| Staurodesmus dickei (Ralfs) S. Lillieroth | - | NR | + | R |
| | Staurodesmus cf. subulatus (Kütz.) Thom. var. americana (Scott & Grönbl) Teil. | - | NR | + | R |
| | *Desmidium swartzii Ag. | + | R | + | R |
| | Desmidium sp. | + | R | + | R |
| | *Spondylosum tetragonum West | - | NR | + | R |
| | *Xanthidium raciborskii Gutw. | + | R | + | R |
| | Xanthidium urniforme (W. West & G. S. West) Scott et
Croasdale | - | NR | + | R |
| | Xanthidium sp. 1 | + | R | + | R |
| | Xanthidium sp. 2 | - | NR | + | R |
| | Xanthidium subtrilobum W. West & G. S. West var.
africanum (Schmidle) Grönbl. & Scott | + | R | + | R |
| Euglenophyceae | *Astasia torta Pringsheim | - | NR | + | R |
| Euglenaceae | Euglena allorgei Defl. | + | R | + | R |
| *Euglena clavata Skuja | + | R | + | LF |
| | Euglena sp. 1 | + | R | + | R |
| | *Euglena ehrenbergii Klebs | + | R | + | R |
| | *Euglena proxima Dang. | + | F | + | F |
| | Euglena sp. 2 | - | NR | + | R |
| | *Euglena gaumei Allorge & Lefèvre | - | NR | + | R |
| | Euglena tripteris (Duj.) Klebs | + | R | + | R |
| *Euglena texta (Duj.) Hübn. | + | R | + | R |
| Lepocinclis acus Marin et Melk. | + | F | + | LF |
*Lepocinclis acus Marin et Melk. var. detonii (van Oye)
Huber-Pest. | + | R | + | R |
| *Lepocinclis fusiformis (Cart.) Lemm. | + | R | + | R |
| Lepocinclis marssonii Lemm. | - | NR | + | R |
| *Lepocinclis ovum (Ehr.) Lemm. | - | NR | + | R |
| *Lepocinclis oxyuris Marin et Melk. | + | R | + | R |
| | Lepocinclis oxyuris Marin et Melk. var. charkowensis
(Swir.) Chu. | - | NR | + | R |
| | Lepocinclis spirogyroides Marin et Melk. | + | R | - | NR |
| | *Lepocinclis spirogyroides Marin et Melk. var. fusca (Klebs) Kosmala & Zakrys | - | NR | + | R |
| | *Lepocinclis teres (Schmitz) Francé | - | NR | + | R |
| | *Monomorphina arnoldi (Swir.) Marin et Melk. | - | NR | + | R |
| | Phacus acuminatus Stokes | + | R | + | LF |
| *Phacus angulatus Pochm. | + | R | + | R |
| *Phacus anomalus Fritsch & Rich | - | NR | + | R |
| | Phacus curvicauda Swir. | + | R | + | R |
| | *Phacus glaber (Defl.) Pochm. | + | R | + | R |
| | *Phacus limnophila Linton et Karnk. | - | NR | + | R |
| Phacus longicauda (Ehr.) Duj. | + | R | + | R |
| Phacus longicauda (Ehr.) Duj. var. rotunda (Pochm.)
Hub.-Pest | + | R | + | R |
| Phacus meson Pochm. | - | NR | + | R |
| *Phacus onyx Pochm. | - | NR | + | R |
| Phacus orbicularis Hübn. | + | R | + | R |
| *Phacus pusillus Lemm. | - | NR | + | R |
| *Phacus platalea Drez. | - | NR | + | R |
| *Phacus pleuronectes (O. F. Müll) Duj. | + | R | + | R |
| Phacus sp. 1 | - | NR | + | R |
| Phacus sp. 2 | + | R | + | R |
| *Phacus suecicus (Lemm.) Lemm. | + | R | + | R |
| | Phacus tortus (Lemm.) Skvortz. | + | R | + | R |
| | Strombomonas deflandrei (Y. V. Roll) Defl. | + | R | + | R |
| | Strombomonas ensifera (Daday) Defl. | + | R | + | R |
| Strombomonas girardiana (Playf.) Defl. | - | NR | + | R |
| | Strombomonas girardiana (Playf.) Defl. var. glabra Playf. | - | NR | + | R |
| | Strombomonas guinkoi Zongo, Couté et Masc. | - | NR | + | R |
| | Strombomonas jaculata (Palmer) Defl. | - | NR | + | R |
| | Strombomonas lanceolata (Playf.) Defl. | + | R | + | R |
| | Strombomonas maxima (Skvortz.) Defl. | + | R | + | R |
| | *Strombomonas treubii (Wolosz.) Defl. | + | R | + | R |
| | Strombomonas verrucosa (Daday) Defl. | + | LF | + | R |
| | *Strombomonas verrucosa (Daday) Defl. var. claviformis
Defl. | - | NR | + | R |
| | Strombomonas verrucosa (Daday) Defl. var. zmiewika
(Swir.) Defl. | - | NR | + | R |
| | *Trachelomonas allorgei Defl. | - | NR | + | R |
| | *Trachelomonas armata (Ehr.) Stein | - | NR | + | R |
| | *Trachelomonas armata (Ehr.) Stein var. coronata Defl. | - | NR | + | R |
| | *Trachelomonas armata (Ehr.) Stein var. ovata Swir. | - | NR | + | R |
| | *Trachelomonas bacillifera Playf. | + | R | + | R |
| | *Trachelomonas bernardinensis Vischer var. africana Defl. | - | NR | + | R |
| | *Trachelomonas dubia (Swir.) Defl. | + | R | + | R |
| | Trachelomonas hispida (Perty) Stein & Defl. var. coronata
Lemm. | + | R | + | R |
| | *Trachelomonas raciborskii Wolle | + | R | + | F |
| | *Trachelomonas scabra Playf. var. scabra | - | NR | + | R |
| | Trachelomonas sp. 1 | - | NR | + | R |
| | Trachelomonas sp. 2 | - | NR | + | R |
| | Trachelomonas sp. 3 | - | NR | + | R |
| | Trachelomonas sp. 4 | - | NR | + | R |
| *Trachelomonas superba Swir. & Defl. | - | NR | + | R |
| *Trachelomonas volvocina Ehr. | + | LF | + | LF |
| | Trachelomonas volvocinopsis Swir. | + | R | + | F |
| Bacillariophyceae | *Frustulia rhomboïdes (Ehrenb.) De Toni | + | R | + | R |
| Amphipleuraceae |
| Bacillariophyceae | *Diploneis minuta (Petersen) Cleve | + | R | + | R |
| Bacillariaceae | *Hantzschia amphioxys (Ehrenb.) Grun. | + | LF | + | R |
| Nitzschia sp 1 | - | NR | + | LF |
| Nitzschia sp 2 | + | R | - | NR |
| Bacillariophyceae | Gomphonema sp. | + | R | + | R |
| Gomphonemataceae |
| Bacillariophyceae | Eunotia pectinalis (Kütz.) Rabh. | + | R | + | LF |
| Eunotiaceae |
| Bacillariophyceae | Navicula sp. | - | NR | + | F |
| Naviculaceae |
| Bacillariophyceae | Pinnularia sp. 1 | - | NR | + | R |
| Pinnulariaceae | Pinnularia sp. 2 | + | R | - | NR |
| *Pinnularia divergens W. Smith | + | R | + | F |
| *Pinnularia gibba Ehr. | + | R | + | LF |
| *Pinnularia microstauron (Ehr.) Cleve | + | R | + | R |
| Pinnularia sp. 3 | + | LF | + | R |
| Bacillariophyceae | *Stauroneis acuta W. Smith | + | R | + | R |
| Stauroneidaceae | *Stauroneis nobilis Schum. | + | R | + | R |
| *Stauroneis phenicenteron (Nitz.) Ehr. | - | NR | + | R |
| Stauroneis sp. | + | R | + | F |
| Coscinodiscophyceae | Aulacoseira granulata (Ehr.) Ralfs | - | NR | + | R |
| Melosiraceae |
| Dinophycideae | Peridinium sp. 1 | - | NR | + | R |
| Peridiniaceae | Peridinium sp. 2 | - | NR | + | R |
| Glenodinium sp. | - | NR | + | R |
Note: (-): absent, (+): present, (*): species recorded for the first time in Burkina Faso, NR: non recorded, R: rare, F: frequent, LF: less frequent, all the different species are represented as figures in Zongo [9].
References
- Zongo B., Boussim I. J. (2015). The effects of physicochemical variables and tadpole assemblages on microalgal communities in freshwater temporary ponds through an experimental approach. Aquatic Biosystems, 11 (1): 1-14.
- Loiselle S. A., Cózar A., Dattilo A., Bracchini L., Cálvez J. A. (2007). Limitation to algal growth in tropical systems. Freshwater Biology, 52: 305-12.
- Chindah A. C. (2004). Response of periphyton community to salinity gradient in tropical estuary, Niger Delta. Polish Journal of Ecology, 52: 83-89.
- Reynolds C. S. (2000). Phytoplankton designer – or how to predict compositional responses to trophic – state change. Hydrobiologia, 424: 123 - 132.
- Kelly M. G., Whitton B. A. (1995). The Trophic Diatom Index: a new index for monitoring eutrophication in rivers.Journal of Applied Phycology, 7: 433-444.
- Fore L. S., Grafe C. (2002). Using diatoms to assess the biological condition of large rivers in Idaho (U.S.A.).Freshwater Biology,47: 2015–2037.
- Zongo B., Zongo F., Toguyeni A., Boussim I. J. (2016). Water quality in forest and village ponds in Burkina Faso (Sub-Saharan Africa). Journal of forestry research, accepted.
- Shashi Shekhar T. R., Kiran B. R., Puttaiah E. T., Shivaraj Y., Mahadevan K. M. (2008). Phytoplankton as index of water quality with reference to industrial pollution. Journal of Environmental Biology, 29 (2): 233-236.
- Zongo B. 2011. Communautés micro-algales dans les mares temporaires, interactions avec variables physico-chimiques et assemblage de têtards en Afrique de l’Ouest. Thèse de Doctorat, Univ. Ouaga., Burkina Faso, 192 p.
- Zongo B., Zongo F., Ouattara A., Boussim IJ. (2011). A taxonomic study of the genus Closterium Nitzsch (Zygnematophyceae, Streptophyta) in temporary ponds (Burkina Faso, West Africa). Cryptogamie Algologie, 32 (3): 255-270.
- algaebase.org. Data base of information on algae that includes terrestrial, marine and freshwater organisms. Visited in 2016.
- Maurer K., Durka W., Stöcklin J. (2003). Frequency of plant species in remnants of calcareous grassland and their dispersal and persistence characteristics. Basic and Applied Ecology, 4 (4): 307-316.
- Ouattara A., Podoor N., Teugels G. G., Gourène G. (2000). Les micro-algues de deux cours d’eau (Bia et Agnébi) de Côte d’Ivoire.Systematics and Geography of plants,70: 315-372.
- Bourrelly P. (1990). Les algues d’eau douce - Les algues vertes. Tome I, édit. N. Boubée, 572 p.
- Van den Hoek C., Mann D. G., Jahns H. M. (1995). Algae: An introduction to phycology. Cambridge University Press, Cambridge, UK, 455 p.
- de Reviers B. (2002). Biologie et phylogénie des algues. Tome 1, edit. Belin, Paris, 353 p.
- White F. (1986). La végétation d’Afrique: mémoire accompagnant la carte de végétation de l’Afrique. ORSTOM, Paris (France), 384 p.
- Ouattara A., Podoor N., Gourène G. (2001). Etudes préliminaires de la distribution spatio-temporelle dans un système fluvio-lacustre africain (Bassin, Côte d’Ivoire).Hydroecol. Appl., 13 (1): 113-132.
- Zongo F., Zongo B., Ouéda A., Boussim I. J., Couté A. (2008). Nouveaux taxa de micro-algues dulçaquicoles pour le Burkina Faso (Afrique de l’Ouest): II- Cyanoprocaryota, Heterokontophyta, Euglenophyta.Annale Univ. Ouaga., 5: 51-78.
- Compère P., Iltis A. (1983). The phytoplankton. In: Lake Chad. Ecology and productivity of a shallow tropical ecosystem. Ed. Carmouze J-P., Durand J. R., C. Lévêque. Junk Publ., The Hague: 145-197.
- John D. M. (1986). The inland waters of tropical West Africa. An introduction and botanical review. Arch. Hydrobio., Beih. E. Limnol., 23: 1-244.
- Kadiri M. O. S. (1993). Records of members of the genus Cosmarium Corda ex Ralfs (Desmidiaceae, Chlorophyta) in shallow West African reservoir.Nova hadwigia, 57 (1-2): 109-122.
- Zongo F. (2007). Inventaire et systématique des micro-algues dulçaquicole du réservoir de Bagré au Burkina Faso (Province du Boulgou). Thèse de doctorat d’Etat, Univ. Ouaga., 208 p.
- Sheath R. G., Wehr J. D. (2003). Freshwater algae of North America: ecology and classification. Academic Press, Amsterdam, the Netherlands, 918 p.
- Iltis A. (1974). Le phytoplancton des eaux natronées du Kanem (Tchad). Influence de la teneur en sels dissous sur le peuplement algal. Thèse de Doctorat d’État, ORSTOM, Paris 6, 420 p.
- Bourrelly P. (1985). Les algues d’eau douce - Les algues bleues et rouges. Edit. N. Boubée, 606 p .
- Smith G. M. (1950). Freshwater algae of the United States, 2nd ed. McGraw-Hill, New York, 719 p.
- Leitão M., Couté A. (2005). Guide pratique des Cyanobactéries planctoniques du Grand Ouest de la France: manuel pour les prélèvements et la reconnaissance à l’usage des gestionnaires des eaux de surface: caractéristiques, échantillonnage, identification. Éd. AESN, Paris, 63 p.
- de Reviers B. (2003). Biologie et phylogénie des algues. Tome 2, edit. Belin, Paris, 255 p.
- Gayral P. (1975). Les Algues: morphologie, cytologie, reproduction, écologie. Doin éditeurs. 165 p.
- Nevo E., Wasser S. P. (2000). Cyanoprocaryotes and algae of continental Israël. Edit. Ruggell, ISBN 3-940144-23-5, 629 p.
- Itlis A. (1980). Les algues. In: Durand J.-R. & Lévêque C. (Eds.): Flore et faune aquatiques de l’Afrique Sahélo-Soudanienne. Tom. I, édit. ORSTOM – Collection Initiation Document techniques n˚ 44, Paris, p 9-61.

 (B. Zongo)
(B. Zongo) 








