| 1. | ||
| 2. | ||
| 2.1. | ||
| 2.2. | ||
| 3. | ||
| 4. | ||
Evaluation of the Suspending Properties of Cyperus Esculentus (Tiger Nut) Starch in Sulphadimidine Suspension
Okorie O., Azaka J. E., Ibeshi E.
Department of Pharmaceutics and Pharmaceutical Technology, Faculty of Pharmaceutical Sciences, University of Port Harcourt, Rivers State, Nigeria
Email address
 (Azaka J. E.)
(Azaka J. E.) Citation
Okorie O., Azaka J. E., Ibeshi E. Evaluation of the Suspending Properties of Cyperus Esculentus (Tiger Nut) Starch in Sulphadimidine Suspension. American Journal of Biomedical Science and Engineering. Vol. 1, No. 1, 2016, pp. 1-7.
Abstract
Starch is a natural polysaccharide polymeric material occurring in the leaves, seeds, stems, tubers and roots of plants. It is one of the most commonly used excipients in pharmaceutical formulations because of its low-cost, relative abundance and inertness. Cyperus esculentus is a perennial herb of both the tropics and temperate regions of the world and is widely cultivated in Nigeria especially in the northern part. Until recently, its use had been limited to food and cosmetic applications. This research is aimed at ascertaining the suitability of C. esculentus starch as a suspending agent in sulphadimidine suspension and to compare its suspending properties with that of compound tragacanth BP (reference). Starch was isolated from C. esculentus tubers and suspensions of sulphadimidine containing C. esculentus starch prepared according to the British Pharmacopoeia formula were compared to those containing compound tragacanth BP at a concentration range of 2%, 4%, 6% and 8% w/v. The sedimentation profile (volume and height), redispersibility, flocculation studies and rheological behaviour of the suspensions were used as evaluation parameters. The results obtained showed that suspensions prepared with C. esculentus starch had superior suspending properties to those formulated with compound tragacanth BP (P<0.05) at all concentrations studied. This study therefore indicates that C. esculentus starch is suitable for use as a suspending agent in pharmaceutical suspensions due to its inherent characteristics.
Keywords
Cyperus esculentus (Tiger Nut), Compound Tragacanth BP, Sulphadimidine Suspension, Suspending Properties, Natural Polysaccharide
1. Introduction
Excipients are pharmacologically inactive substances that are included in pharmaceutical formulations to serve as carriers for the active ingredients [1]. They play a critical role in the manufacture of drugs by helping to preserve the efficacy, safety and stability of active pharmaceutical ingredients (APIs) and ensuring that they deliver their expected benefit to the patients [2]. Excipients can be classified on the basis of their origin, use in dosage form or functions they perform [3]. On the basis of their origin, they can either be of natural or synthetic origin [4]. Those of natural origin are of particular interest to formulation scientists because of their reliability and sustainability, and may be grouped into animal, plant/vegetable and mineral sources [4]. Plant polysaccharides are a group of polymers that are widely used in pharmaceutical formulations and in several cases their presence plays a fundamental role in determining the mechanism and rate of drug release from the dosage form. These naturally occurring polymers have been employed as excipients in the pharmaceutical industry for the formulation of solid, liquid and semisolid dosage forms in which they play different roles as disintegrants, binders, film formers, matrix formers or release modifiers, thickeners or viscosity enhancers, stabilizers, emulsifiers, suspending agents and mucoadhesives [5, 6]. Their growing role and application in the pharmaceutical industry may be attributed not only to the fact that they are biodegradable and toxicologically harmless raw materials of low-cost and relative abundance compared to their synthetic counter parts [7, 8], but also because they are renewable and if cultivated and harvested in a sustainable manner can provide a constant supply of raw material [9].
Starch is a polysaccharide of glucose. This biopolymeric material is a naturally abundant carbohydrate found chiefly in seeds, leaves, roots, fruits, tubers and stem piths of plants [10]. It is a white, amorphous, tasteless powder made up of amylose and amylopectin [11]. Starches can be obtained from maize, cassava, yam, rice, potatoes, wheat, tiger nuts and several other sources. They vary widely in appearance according to their biological origin. Therefore, a thorough investigation of the physicochemical properties is warranted before they are used [12]. Starch has a wide range of uses and is applied in a variety of industries including food, textiles, plastics, adhesives, paper, cosmetics and pharmaceutical [10]. In the pharmaceutical industry, starches have been employed as binders, disintegrants, diluents (fillers), lubricants, glidants, coating and dusting media for tablet coating, production of micro-particles for drug delivery and delivery of orally and intramuscularly administered vaccines etc. [10, 13, 15].
A Pharmaceutical suspension is a coarse dispersion in which the internal phase (therapeutically active ingredient) is dispersed uniformly throughout the external phase. Like other disperse systems, suspensions are thermodynamically unstable and the development of a stable suspension over the shelf life of the drug product continues to be a challenge on many fronts. As a result, it is necessary to include in the dosage form, a stabilizer or suspending agent which reduces the rate of settling and permits easy re-dispersion of any settled particulate matter both by protective colloidal action and by increasing the consistency of the suspending medium [16-18]. The selection of the proper excipients (surfactants, viscosity imparting agent’s e. tc.) is essential in the development of a suitable pharmaceutical suspension because the physical stability of a suspension is mainly dependent on the type of suspending agent rather than the physical properties or characteristics of the API [19]. Suspending agents may be (i) inorganic materials, (ii) synthetic compounds, or (iii) polysaccharides. Starches belong to the latter group.
With the increase in demand for natural products, it has become necessary to explore newer sources of starch to meet industrial demands and also provide cheap and effective natural excipients that can be used as alternatives to the available ones. Cyperus esculentus (tiger nut) is an underutilized crop of the family Cyperaceae which produces rhizomes from the base and tubers that are somewhat spherical. It is commonly known as "earth almond", "chufa", "chew-fa" and "Zulu nuts". It is known in Nigeria as "Ayaya" in Hausa, "Ofio" in Yoruba and "Akiausa" in Igbo. Three varieties (black, brown and yellow) of the crop are cultivated. Among these, only two varieties, yellow and brown, are readily available in the market [20]. The yellow variety is preferred over others because of its inherent properties like its large size, attractive colour and fleshier nature. The yellow variety also yields more milk, contains lower fat and higher protein and less anti-nutritional factors especially polyphenols [21]. Tiger nut can be consumed raw, roasted, dried, baked or made into a refreshing beverage [22]. Until recently, its use had been limited to food and cosmetic applications.
This study aims to investigate the suitability of C. esculentus starch as a suspending agent in Sulphadimidine suspension and to compare its suspending properties with that of Compound tragacanth BP.
2. Materials and Methods
2.1. Materials
Oven (Memmert U-27, Germany), Bench top Centrifuge machine (PEC Medicals, USA) Household blender (Intermet), Muslin cloth, Water Bath, Sulphadimidine powder (Baoji Shanghai), compound tragacanth powder (BDH Laboratory Supplies Poole, England), Amaranth solution (E- Merck Darmstardt Germany), Benzoic acid, Raspberry syrup, Chloroform (Riedel-de Haen, Germany), Ethanol 96% (BDH England), Propylene glycol, Glycerol, Sodium carboxymethyl cellulose (Griffin and Geogy, England) were used for this study. C. esculentus tubers were purchased from Choba Market, Port-Harcourt, Nigeria.
2.2. Methods
2.2.1. Isolation of Starch from Tubers of Cyperus esculentus
Tiger nut tubers were sorted to remove stones, dirt materials and rotten stems before cleaning in water to remove any adhering soil. The cleaned tubers were masticated in a masticating juicer and the resulting chaff blended in batches in a blender with distilled water. The paste was passed through a muslin cloth and the chaff separated from the milk. They were both stored separately. The milk was centrifuged at 3000 rpm for 15 min. The starch was collected and oven dried at 55°C for 180 min. The dried caked starch obtained was blended and passed through a sieve of 1 mm mesh size. It was re-blended and comminuted further in a porcelain mortar before passing through a sieve of mesh size 250 mm. The starch was stored in a container.
2.2.2. Extraction of Oil from Tubers of Cyperus esculentus
The supernatant from the centrifuge obtained above was collected and heated at 60°C for 90 min and 180°C for 5 min. This was done until all the oil was extracted. The oil was stored in a glass jar.
2.2.3. Phytochemical Screening
Preliminary tests were performed to confirm the nature of the starch obtained. The phytochemical tests that were conducted are test for saponin, alkaloid, tannin, phlobatannins, anthraquinones, flavonoids, selivanoff’s, molisch, protein, iodine, keller-killiani and test for cardiac glycoside [23].
2.2.4. Preparation of Sulphadimidine Suspensions
10 g of sulphadimidine powder and 2.0 g of compound tragacanth powder were weighed and triturated in a mortar. 20 ml of Raspberry syrup was added in aliquot proportion and triturated for 3 min until a smooth paste was formed. 2 ml of benzoic acid and 1 ml of amaranth solutions were added gradually with constant stirring and then mixed with 50 ml of chloroform water double strength. The mixture was transferred into a 100 ml amber bottle, made up to volume with distilled water and then shaken vigorously for 2 min (thus making 2% w/v of the gum in the preparation). The procedure was repeated using different concentrations, 4, 6 and 8% w/v of compound tragacanth powder. The above method was also repeated for the same concentrations of tiger nut starch.
2.2.5. Determination of Suspension Properties
i. Physical test
The prepared suspensions were observed for physical properties such as color, odor, taste, texture and fracture at 24-hour intervals for a period of 7 days.
ii. Sedimentation volume
50 ml of each suspension was stored in a 50 ml-measuring cylinder and left undisturbed. The sedimentation volumes of the suspensions were determined at room temperature by measuring the volume of the sediment in the suspensions placed in the measuring cylinders at 24-hour intervals for 7 days. The sedimentation volume, F (%), was then calculated using the following equation:
F = Vu / Vo (1)
%F = [Vu / Vo] × 100/1 (2)
Where, Vu = ultimate volume of sediment; Vo = original volume of sediment before settling occurred; %F = Percentage Sedimentation volume [24].
From the values of F obtained, Graphs of sedimentation volume (Vu/Vo) against time were plotted.
iii. Flocculation studies
100ml suspensions were prepared using compound tragacanth powder and either 0.5, 1.0 or 1.5%w/v sodium carboxymethylcellulose. Another set of 100 ml suspensions were prepared by replacing compound tragacanth powder with tiger nut starch and another set by including 0.5, 1.0 or 1.5%w/v sodium carboxymethylcellulose with 0.8 g of neutral electrolyte (NaCl) into suspensions containing tiger nut starch. A blank set of suspensions were prepared as well. The tubes were covered in paraffin oil to prevent evaporation and heated for 90 minutes. The sedimentation height was measured at 15 minutes interval.
iv. Rheological assessment (Flow Rate)
The time taken for 5 ml sample of suspension to flow through a 5ml pipette was determined and the flow rate calculated using the following equation [25]:
Flow rate = ƞα =![]() (3)
(3)
v. Re-dispersibility test
50 ml of each suspension was poured into six calibrated tubes which were stored at room temperature for 5, 10, 15, 20, 25 and 30 days. At the end of each storage period, one tube was removed and hand-shaken at a moderately constant rate of 20 shakes to re-disperse the sediment formed in the cylinder. The presence of deposit if any, was recorded.
vi. vi. Statistical analysis
One way analysis of variance (ANOVA) was used to determine if there was significant difference in the sedimentation volume of the suspending agents investigated. Mean and Standard deviation for values obtained were determined as appropriate.
3. Results and Discussion
The average yield of starch obtained from the tubers of C. esculentus was 5.298% w/w while the percentage yield of oil obtained from the same source was 0.07%. The results obtained from the morphological and physical tests are presented in Table 1. Phytochemical tests carried out on the starch confirmed the presence of protein, triterpenoid, carbohydrate, alkaloids, starch and reducing sugar. The results of phytochemical screening of the starch are summarized in Table 2.
Table 1. Morphological and Physical characterization of tiger nut starch.
| Parameters | Observation |
| Colour | White |
| Taste | Tasteless |
| Odour | Odourless |
| Texture | Fine |
| Fracture | Smooth |
Table 2. Phytochemical screening of tiger nut starch.
| Tests | Observation |
| Saponin | Absent |
| Flavonoid | Absent |
| Starch | Present |
| Anthraquinone (Borntrager’s test) | Absent |
| Triterpenoid (Lieberman-Burchard’s test) | Present |
| Carbohydrates (Molisch’s test) | Present |
| Tannins (Ferric chloride test) | Absent |
| Phlobatanin | Absent |
| Alkaloids (Mayer’s and Dragendorff) | Present |
| Proteins (Million’s test) | Present |
| Reducing sugar | Present |
Sedimentation volume is the ratio of the ultimate height (Hu) of sediment to the initial height (H0) of the total suspension. The larger this value, the better is the suspendibility [26]. The sedimentation volumes (%F) obtained for the formulations at different concentrations of suspending agent are presented in Table 3 and illustrated in Figure 1 and 2. The results showed that all the suspensions settled to form sediments at various times. This is due to the effect of gravity on the suspended particles. Suspensions containing tiger nut starch were shown to give significantly higher sedimentation volumes than those containing compound tragacanth BP (P<0.05) at all concentrations of prepared samples. This shows that tiger nut starch produces better suspendibility than compound tragacanth BP. It was also observed that the sedimentation volume of the suspensions increased as the concentration of suspending agents was increased. The change in sedimentation volume in all the suspensions was however, minimal throughout the study period.
Table 3. Effect of varying concentration of suspending agents on the sedimentation volume of sulphadimidine suspension.
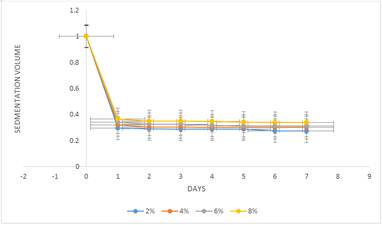
Figure 1. Sedimentation volume (F) against time (days) for different concentrations of tiger nut starch in Sulphadimidine (10%w/v) suspension.
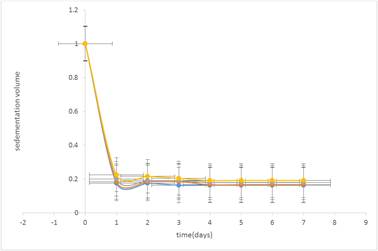
Figure 2. Sedimentation volume (F) against time (days) for different concentrations of compound tragacanth in Sulphadimidine (10%w/v) suspension.
Flocculation is a process of destabilizing the suspending particles in a suspension by adding chemicals called flocculating agents. They include polymers, surfactant and neutral electrolytes. Introduction of a flocculating agent generally improves the performance of the suspending agent [27]. The results of flocculation studies are expressed in Table 4 and Figure 3. The height of sediment formed in suspensions containing tiger nut starch was found to be higher than those formed in suspensions prepared with compound tragacanth BP. This indicates that the suspended particles settled more rapidly in the former than in the latter. This is a desirable feature as particles that settle rapidly are more likely to form loose aggregates (flocs) that can be easily re-dispersed with a minimum of agitation as opposed to particles than settle slowly to form a compact sediment or cake which is difficult to re-disperse by agitation. Sodium carboxymethylcellulose is a cellulose polymer used as a flocculating agent in pharmaceutical suspensions. It acts by increasing the viscosity of the aqueous vehicle, hindering the movement of the suspended particles. It may also form adsorbed layers on the particles, thereby influencing stability through stearic stabilization and in some cases it facilitates bridging between particles, linking them together in a loosely arranged structure [27]. The addition of varying concentrations of sodium carboxymethylcellulose to the suspensions containing compound tragacanth BP increased the sedimentation height at a low concentration (0.5%). However, at higher concentrations, the sedimentation height reduced until a minimum height of 0 cm was obtained at a concentration of 1.5%. For suspensions containing tiger nut starch, the sedimentation height reduced at all concentrations of added sodium carboxymethylcellulose. This result confirms the effectiveness of sodium carboxymethylcellulose as a flocculating agent in pharmaceutical suspensions.
The flow rate of the various concentrations of sulphadimidine suspension formulated with tiger nut starch as well as that of suspensions containing compound tragacanth BP are presented in Table 5. An inverse relationship between concentration of suspending agent and flow rate was observed for all the suspensions i.e. the flow rate decreased with increasing concentration of suspending agent. This is due to the increase in the viscosity of the medium that occurs as a result of increased concentration of the polymers which tends to hinder flow.
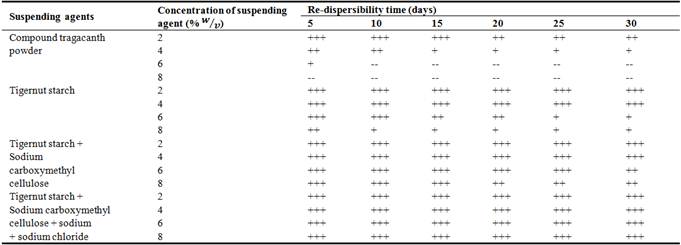
Suspensions tend to settle with time, hence, they are designed to be easily re-dispersed by minimum agitation to produce a homogenous suspension which ensures uniformity of dosage. Re-dispersibility is a function of the nature of the suspension (flocculated or deflocculated). The re-dispersibility of the suspendants are shown in Table 6. It was observed that suspensions prepared with tiger nut starch were easier to re-disperse than those containing compound tragacanth powder. This is due to the superior suspending ability of the former compared to the latter. The addition of the flocculating agent sodium carboxymethylcellulose and the electrolyte sodium chloride improved the ease re-dispersibility of the suspensions.
Table 4. Flocculation studies.
| Suspending agent (height in cm) at 15-min intervals | 0 | 15 | 30 | 45 | 60 | 75 | 90 |
| A | 0 | 1.0 | 1.0 | 1.0 | 1.0 | 1.0 | 1.0 |
| B | 0 | 6.9 | 6.9 | 6.2 | 6.2 | 6.2 | 6.2 |
| A + 0.5% Sodium carboxymethylcellulose | 0 | 1.5 | 1.5 | 1.5 | 2.0 | 2.0 | 2.0 |
| A + 1.0% Sodium carboxymethylcellulose | 0 | 0.3 | 0.3 | 0.5 | 0.8 | 0.8 | 0.8 |
| A + 1.5% Sodium carboxymethylcellulose | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| B + 0.5% Sodium carboxymethylcellulose | 0 | 1.5 | 1.7 | 1.7 | 2.0 | 2.0 | 2.0 |
| B + 1.0% Sodium carboxymethylcellulose | 0 | 0.5 | 0.6 | 1.0 | 1.0 | 1.0 | 1.0 |
| B + 1.5% Sodium carboxymethylcellulose | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| B + 0.5% Sodium carboxymethylcellulose + 0.8 g Sodium chloride | 0 | 4.0 | 3.1 | 3.0 | 3.0 | 3.0 | 3.0 |
| B + 1.0% Sodium carboxymethylcellulose + 0.8 g Sodium chloride | 0 | 4.8 | 3.8 | 3.2 | 3.0 | 3.0 | 3.0 |
| B + 1.5% Sodium carboxymethylcellulose + 0.8 g Sodium chloride | 0 | 9.3 | 8.0 | 6.0 | 5.0 | 5.0 | 5.0 |
Key: A represents suspension containing compound tragacanth powder
B represents suspension containing tiger nut starch
Table 5. Effect of varying concentration of suspending agent on the flow rate (ml/s) of sulphadimidine suspension.
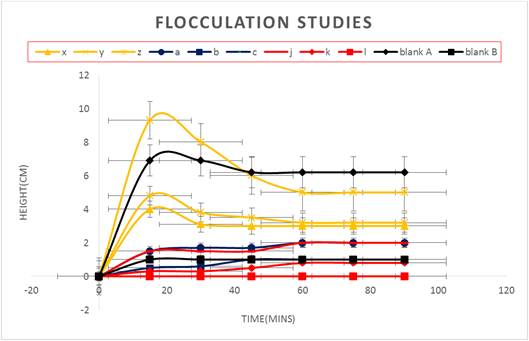
Figure 3. Graph of Flocculation studies.
Table 6. Redispersibility of suspensions after shaking 20 times over a period of 30 days.
4. Conclusion
The study revealed that Sulphadimidine suspensions formulated with tiger nut starch as the suspending agent were more stable than those containing Compound tragacanth powder BP. This is as a result of the superior suspending ability of tiger nut starch when compared to Compound tragacanth BP. The suspending property of tiger nut starch can however be enhanced by the addition of Sodium carboxymethylcellulose and the electrolyte Sodium chloride which function as flocculating agents to maintain the particles in a dispersed state and facilitate re-dispersibility.
It can therefore be concluded that starch from Cyperus esculentus (tiger nut) which is widely cultivated in several regions of the world and can be cheaply sourced has potential use as suspending agent in pharmaceutical suspensions.
References