Germination, Desiccation and Storage of Ardisia virens var. annamensis Seeds
Qihe Yang1, Qinying Lan2, Yunhong Tan2, Xiaojuan Yin1
1School of Life Sciences, Jiaying University, Meizhou, The People’s Republic of China
2Tropical Plant Germplasm Bank, Xishuangbanna Tropical Botanical Garden, the Chinese Academy of Sciences, Menglun, Mengla, The People’s Republic of China
Emai address

(Qihe Yang)
Citation
Qihe Yang, Qinying Lan, Yunhong Tan, Xiaojuan Yin. Germination, Desiccation and Storage of Ardisia virens var. annamensis Seeds. International Journal of Biological Sciences and Applications. Vol. 3, No. 4, 2016, pp. 42-49.
Abstract
Ardisia virens var. annamensis is a famous ornamental species. Due to deforestation and destructive overexploitation, its natural population size has been greatly reduced in recent years. To provide information for its basic genetic conservation and expanding its population by artificial breeding, the germination, desiccation-tolerance and storage lifespan of the seed were studied. The field investigation found in the natural environment the plant had low seed yield. The indoor seed storage and germination experiment showed that the germination temperature ranged from 4°C to 30°C, and the seeds could germinate in full light and in the dark. At temperatures above 20°C, light had no significant effect on germination, but at temperatures between 10-15°C, light significantly promoted germination. The mature seeds had relatively moisture content and weak desiccation-tolerance, so their storage behaviour should be classified as recalcitrant. The seeds remained viable longer in moist storage than in dry storage. These responses of seed germination to light, temperature and desiccation indicate that this shrub have relatively strong adaptability to the natural tropical and subtropical moist environments in South China, the change environment caused by forest fragmentation may be one of important reasons for decreasing of the population.
Keywords
Seed, Germination, Desiccation-Tolerance, Storage Longevity, Ardisia virens var. annamensis
1. Introduction
The pan-tropical genus Ardisia (Myrsinaceae) contains approximately 400–500 species of evergreen shrubs distribute in the subtropical and tropical regions around the world [1]. Some Ardisia species have been used for medicinal purposes, because they contain a wide array of biologically active phytochemical constituents such as bergenin and ardisin [2]. The Ardisia genus has a stunning appearance, hardy body and clustered berries. It can flourish both indoors as well as outdoors under suitable conditions, but it susceptible to low temperatures and in this regard mulching around the plants helps considerably in its growth. The species is also not drought tolerant.
In China, Ardisia plants are used not only for landscaping, but also for traditional medicine. But due to overexploitation of wild Ardisia species, it has become increasingly important to conserve the germplasm of this valuable genus. The present article reviews the usage and biological activities of Ardisia compounds, as well as recent progress regarding the use of this genus in clinical researches. The information presented here also illustrates the potential of the genus as a source of therapeutic agents [3].
Ardisia virens Kurz var. annamensis Pitard is an evergreen shrub with attractive bright berries and color petals which are white or light yellow at first but later turn red during the flowering period. It is usually distributed in the sparse woodlands on the top of mountains at an elevation of 900 m in Hainan, Yunnan, Guizhou, Guangxi and Taiwan Province of China and Vietnam. This species is usually regarded as a variant of Ardisia virens, because it was significantly different from Ardisia virens in that its leaves are blade lanceolate, 19-26 cm in length and 2.5-5 cm in width and have dense margin glandular dots in sepals and sparse smooth dots in fruit. Its inflorescences are terminal compound umbels, glabrous, on specialized lateral branches and flowers are papery, white or pink, 7-8 mm, and fruits are red or blackish red, globose, densely black punctuate [1].
Some Ardisia plants thrive in productive, well drained soils with partial to heavy shade in the understory of mesic forests and the growth rates of these shrubs have been positively correlated with soil phosphorus levels [4-5]. These plants are easily propagated by seed or cutting, but only established by seed in the wild. They reach the reproductive maturity around 20cm in height when lateral branches start developing, and vivaciously resprouts after cutting or fire [6].
In germplasm conservation, seeds are critical as they are the major unit of plant reproduction and can be cost-effective for storage, reintroduction and wild population restoration in comparison to preserving genetic material as ex situ living collections. Cryopreservation is an efficient and feasible means of long-term storing orthodox seeds. For successful cryopreservation, seeds have to be desiccated to an optimum level and the protocol for cryopreservation needs to be established. The drying rate can significantly impact the success of cryotolerance and this can be species-specific. Flash-drying or rapid drying is known to improve desiccation tolerance of seeds that are desiccation-sensitive compared with slow drying. Wesley-Smith et al. (2014) suggested that rapidly dried cells retain a greater ability for cell repair upon rehydration [7]. However, Finch-Savage et al. (2006) showed that rapid desiccation could negatively affect germination [8]. More studies are needed to investigate the relationship between desiccation rate and subsequent storage life. Further, little is known about desiccation and storage longevity of seeds of the Ardisia genus. The aim for this study was to establish storage protocols for storing the seeds of these species.
2. Materials and Methods
Most of mature fruits of the shrub were collected manually from more than 30 individual shrubs between June and December 2011 from semi-natural forests, and a small portion of those collected from Xishuangbanna Tropical Botanical Garden in Mengla County, Xishuangbanan Dai Nationality Autonomous Prefecture (21°09’-22°36’N and 99°58’-101°50’ E) in Yunnan Province where the average rainfall is 1200-1600 mm; temperature, 18-22°C; and relative humidity, 77-87%; annual sunshine hours, 1700-2300 hours. The seeds were immediately isolated from the fruits, and air-dried at room temperature (18-23°C) for 3 days, then stored in air-permeable plastic bags at 15°C storage room for 1 month. The seeds were randomly divided into 8 sub-lots and used in the various experiments described below.
2.1. Observation and Determination of Fruit and Seed Characteristics
Fruit characteristics (shape, size, color) were recorded and moisture content (MC) determined gravimetrically after oven drying at 103 ± 2°C for 17h. Similarly, seed characteristics (size, color) were recorded and MC determined after fruit pulp was removed and seeds were manually cleaned (Table 1).
Table 1. The traits of mature fruit and seed of Ardisia virens var. annamensis.
| Pericarp color | Red |
| Fruit Length (mm) | 10.45±0.48 |
| Fruit Diameter (mm) | 9.86±0.43 |
| Seed Length (mm) | 6.98±0.33 |
| Seed Diameter (mm) | 6.97±0.27 |
| 1000-fruit fresh weight | 474.64±8.28 |
| 1000-seed fresh weight | 195.11±2.34 |
| moisture content (fresh) | 50.58±7.59 |
| Flowering period | May - July |
| Fruit ripe period | October - December or next January |
Data in the table are mean ± S. D.
2.2. Seed Size, Weight and Moisture Content
Quadruplicates of 1000 seeds were weighed and 60 seeds were measured immediately after collection with electronic balances and calipers to ascertain the average seed weight and size. Seed MC (moisture content) tests were conducted according to the Rules of ISTA (1993) [9].
2.3. Desiccation Procedure
Initial MC was determined immediately after extraction from fresh fruits. The seeds were desiccated using three methods and each treatment had five durations (12h, 24h, 48h, 72h, and 96h). Firstly, seeds were desiccated by placing them in activated silica gel at 25 ± 2°C, and 4% relative humidity in closed jars with the silica to seed ratio of 18:1 by volume (rapid desiccation). The silica was reactivated every 24 h. In the slow desiccation method, seeds were suspended above saturated NaCl solution in closed jars in 25±2°C and 75% RH (8d, 16d, 45d, 64d). Lastly, the seeds were air-dried under room conditions at 27±2°C and RH approximately 60%. For each desiccation method and duration, a subset of the seeds was used to determine the MC as described above. Eighty seeds for each desiccation method and duration were then germinated in four replicate Petri dishes (each containing 20 seeds) to determine desiccation tolerance. Seeds were germinated in incubators (RXZ-300B, Ningbo Southeastern Equipment Co. Ltd., Ningbo, China) at 25°C with 24 h photoperiod (light level of 2800 lx) in 0.8-1.0% agar medium. Germination was monitored for 40 d.
2.4. Seed Germination Tests
Only full seeds were selected and used for germination tests. Fresh seeds were used to evaluate the effects of temperature, light, MC and storage on seed germination. Quadruplicate samples of 50 seeds were disinfected for 10 min with 0.2% sodium hypochlorite, and then washed with distilled water and finally assigned in 90mm-diameter Petri-dishes with 0.8-1% agar and incubated as above. Germination and emergence was defined as the appearance of a radicle over 0.5 cm in length. The illumination was provided by cool white fluorescent lamps when in photoperiod for each germination treatment. But seed germination was observed every day in the weak indoor natural light, the GP and GI were calculated and recorded and germination experiment lasted 21 d.
Germination at various temperatures Seeds were germinated in incubators under two different light conditions (12 h light of 800-1000 lx and 12 h full dark, 24 h in full dark) at 5, 10, 15, 20, 25, 30, 35°C and AT (ambient Temperature). Testa-striped seeds were germinated at 25°C to evaluate the effect of testa on germination.
Germination at various light conditions Seeds were germinated in incubators of 10, 15, 20, 25, 30, 35, 40°C and AT.
2.5. Germination and Emergence Percentage, Germination and Emergence Index
GP (%)=number of germinated seeds/number of total sampled seeds×100.
GI=∑(Gt/Dt), Gt means numbers of germinated seeds after t d.
EP (%)=number of emerged seedlings /number of total sampled seeds×100.
EI (emergence index)=∑(Gt/Dt), Gt means numbers of emerged seedlings after t d.
2.6. Seed Storage Tests
Seeds were stored by two methods viz. moist storage and dry storage. Fresh seeds in moist storage were firstly mixed in moist sand and then placed into wide-mouth bottles in an incubator of 0-4°C, but those in dry storage were placed onto a plastic screen in an air-ventilated room. After being stored for 90 days, the seeds were taken out to germinate at 25°C with 12 h daily illumination of 800-1000 lx. The freshly harvested seeds were regarded as control.
2.7. Determination of Leakage Rate of Electrolyte
Triplicates of 20 seeds per treatment were soaked in 50 ml double-distilled water for 24 h at ambient temperature, boiled at 100°C for 30 min and then cooled to ambient temperature. The leachate conductivity was determined by an electrical conductivity determinator (DDS-11A; Shanghai No. 2 Analytical Instrument Co., China) at 30±2°C. Relative electric conductivity = electric conductivity before boiling/electric conductivity after boiling.
2.8. Statistical Analysis
Based on successful germination the interaction between the desiccation method and drying were tested. This was modeled using a generalized linear model with a binomial distribution function using R project [version 2.15.1] and Epicalc package. A separate analysis to examine the effect of the desiccation method and desiccation duration on germination by measuring success in germination on seeds stored for different duration at different temperatures was also conducted. Multiple comparisons were used to test within treatment differences after adjusting for α = 0.05 using Tukey test in the multicomp package of R.
Results of germination experiments are expressed in percentage or index (±SD). GP were transformed into angular values and subjected to ANOVA in Microsoft (R) Excel version 2000 (Microsoft, Inc., 1985–1999) to determine if significant differences were present among means and LSD test was used to determine if significant differences occurred between individual treatments.
3. Results
3.1. The Characteristics of Fruit and Seed
The characteristics of mature fruits and seeds were described as follows (Table 1). Ardisia virens var. annamensis flowered from May to July, and the fruits ripened from October to next January, but the flowering and fruit maturity period were slightly different with the different habitats. The vast majority of fruits fell from the mother shrubs until March in next year in South-west China. Field observations showed significant differences in fruit production between different mother shrubs and the different habitats. In organic-rich lowland rain forest valleys, fruit production per mature individual was usually 200 or more, while at higher elevations it was only 50-100 or less, but under the cultivation conditions in gardens, it was 300-400 or more. Our field survey revealed that the fruit production increases with soil fertility and water availability. All the fresh fruits were berry-like drupes. The morphological characteristics of fruits and seeds for this plant are shown in Table 1. After the pericarp brokedown, the seeds dehisced. Most of seeds were flat, oblong or ovate, and the testa of mature seeds was greyish-white with a white stripe.
3.2. Germination at Various Temperatures
The seeds had rod-shaped haustorial cotyledons (0.5-1mm wide, 2.2-3mm long) that remained inside the endosperm and showed hypogeal germination. The seeds germinated most rapidly with fluctuating temperatures of 10-25°C, and reached GP (germination percentage) 100% after a week, but at 4°C, 10°C, 25°C, 30°C and 15-30°C, they reached the highest germination percentage after 2-3 weeks. No seed germinate at temperatures above 35°C. The optimal temperature range for germination is therefore 10~25°C. At optimal temperature, the seeds mostly germinated in the first week during germination experiment, i.e., the seeds required less days to germinate at 10~25°C than at other temperatures (Table 2).
Table 2. Effect of temperature on germination of Ardisia virens var. annamensis.
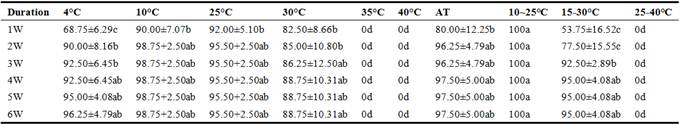
GP: Germination percentage; GI: germination index; AT: ambient temperature (15-24°C/24-35°C, night/day). The values marked with the same letter (a–c) in the same line are not significantly different at 5% level.
3.3. Germination Percentage and Index After Desiccation and Storage
The MC of freshly harvested mature seeds of Ardisia virens var. annamensis was about 50% and GP was approximately 100%. After desiccation for 12 and 24 h with the rapid method, seed MC was reduced to about 42% and 39%, the GP was 100% and 96.25% respectively, and was almost equivalent to that of the fresh seeds. But the GP was declined to below 80% when seed MC was reduced to 28.35%, and was 0% when MC was further reduced to below 9%. The GP were all 0% when seed was stored at -20°C for 1, 2 and 4 months. The seeds stored at the higher temperatures (10°C and 15°C) with the higher MCs had higher GP than the control. For the desiccated seeds with the similar MC, those desiccated by rapid desiccation method had higher GP than those dried by the slow method (Table 3). When at the moisture of about 41%, the seeds dried by the rapid method had a higher germination percentage than those dried by the slow method. The seeds with 50% MC stored at 4-15°C had a relatively long storage life compared with those with MC below 42% and stored at 10°C and 15°C. For the seeds with the similar MC, rapid desiccation is more effective to extend the storage life than slow desiccation. The seeds lose their germinability when the MC was reduced to below 10%, and slight desiccation would significantly shorten the storage life when seed MC was reduced to below 30%, and most of them lose germinability after 3-month storage (Table 3).
Table 3. Germination of Ardisia virens var. annamensis seeds after storage at different temperatures.
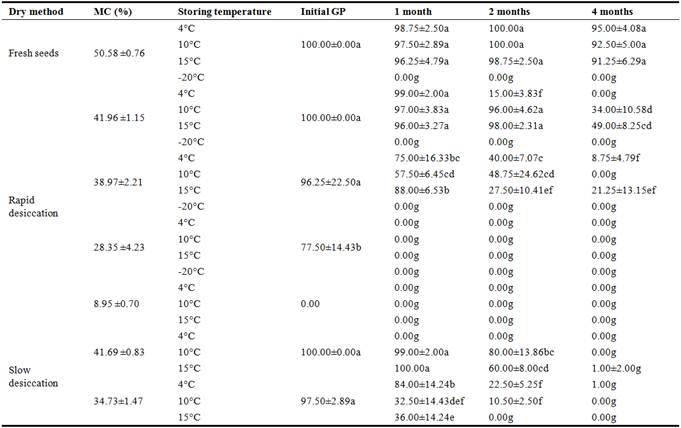
Note: MC means moisture content (%); GP means germination percentage.
3.4. Effect of Light on Seed Germination
There was no significant difference in germination percentage when germination took place at 20°C and above, while at 10°C and 15°C, the germination percentage in the light was significantly higher than that in dark. The GP of testa-stripped seeds was almost the same as that of intact seeds, so it may be concluded that the testa had no significant effect on GP.
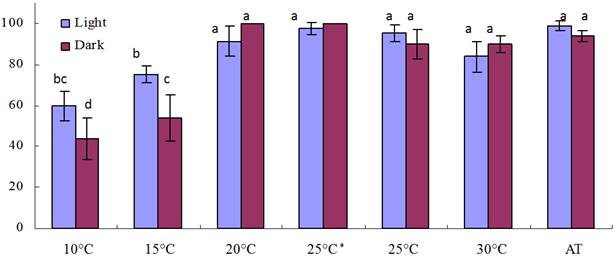
Note: * the testa-stripped seeds were only used here, and the other seeds were all intact.
Figure 1. Germination of Ardisia virens var. annamensis in the light and dark.
3.5. Electric Conductivity of Seeds After Desiccation
The EC (electric conductivity) of seed leachate rose with increasing desiccation duration whether by the rapid or the slow method. However, the rate of desiccation did have a significant effect on the EC of the seed leachate (Table 4). At similar low moisture contents, the EC of the seed leachate obtained through slow desiccation was higher than that through obtained by rapid desiccation. This may be due to the longer time taken to dry the seed was associated with seed ageing. In this study, the appropriate osmotic regulation treatment was not carried out prior to the EC determination experiment, which suggesting rapid desiccation maybe brought less damage to seed microstructure than slow desiccation.
Table 4. Electric conductivity of seed leachate after desiccation of Ardisia virens var. annamensis.
| Seedlot | Moisture content | Electric conductivity |
| CK | 50.58±0.76a | 14.65±2.43e |
| FD12h | 46.15±0.80b | 21.11±3.56d |
| FD1d | 43.56±1.29bc | 40.75±6.28c |
| FD2d | 38.52±0.80c | 66.31±5.84ab |
| FD3d | 39.70±3.30c | 78.85±3.35a |
| SD8d | 35.86±1.75c | 46.16±3.66c |
| SD16d | 25.18±0.96d | 60.89±5.13b |
| SD45d | 9.57±0.03e | 73.42±7.37a |
| SD64d | 8.11±0.12f | 76.94±4.95a |
Table 5. Length and dry weight of seedlings of Ardisia virens var. annamensis.
| | 15d | 30d |
| | Length | Dry weight | Length | Dry weight |
| RD2T 4°C | 4.87±0.72c | 0.48±0.29b | 6.02±0.61b | 0.52±0.09b |
| RD2T 10°C | 4.36±0.99c | 0.22±0.03c | 6.31±1.49b | 0.46±0.14b |
| RD2T 15°C | 2.99±0.69d | 0.17±0.05c | 6.36±0.52b | 0.46±0.10b |
| OH4°C | 7.70±0.29a | 0.90±0.06a | 8.38±0.25a | 0.86±0.18a |
| OH10°C | 7.06±0.99a | 0.83±0.15a | 8.48±1.66a | 1.01±0.28a |
| OH15°C | 7.04±0.78a | 0.66±0.14b | 8.73±1.10a | 1.01±0.11a |
Note: RD means rapid desiccation; OH means the non-desiccated seeds.
3.6. Length and Dry Weight of Seedlings
There were significant differences in length and dry weight of the seedlings obtained from fresh seeds and desiccated seeds. The length and dry weight of desiccated seeds were significantly lower than those of fresh seeds. The desiccation brought some damage on seedling growth. There was no significant difference of length and dry weight at 30 d in the seedlot at between different temperatures. But at 15 d, for the desiccated seeds by the rapid desiccation, the length and dry weight at 15°C was significantly less than those at 4°C and 10°C (Table 5).
4. Discussion
The field survey showed that in the natural or semi-natural forest of Southern Yunnan, China, most Ardisia species, including A. virens var. annamensis populations usually grew in shaded areas of dense evergreen broad-leaved forests, hillsides, dark damp places and valleys. A significant observation was that few seedlings were found in the natural habitats. Compared with records of previous surveys [10], due to the deforestation of a large number of natural or semi-natural forest and utilization as economic forests through planting rubber, banana, litchi, etc, the habitat area suitable for the growth of this shrub has been shrinking. In addition, the populations have been rapidly shrinking probably because of excessive digging the maternal individuals used as traditional Chinese medicine. Although the adult maternal individuals can produce relatively copious quantities of viable seeds under artificial cultivation conditions, they do not produce many seeds in the natural environments.
4.1. Effects of Temperature and Light on Seed Germination
Many tropical plants need high temperatures to promote germination [11]. In this study, most seeds of A. virens var. annamensis could germinate over a temperature range from 4°C to 30°C, and they showed a higher germination percentage between 10°C and 25°C. However, it has been found that other Ardisia species, including A. crenata and A. japonica, germinated well at a temperature range from 20°C to 30°C [12-13]. A. virens var. annamensis seed germination differs from other typical tropical plants [11], in that the majority of seed germinated after 3-4 weeks even at 4°C and 15°C, but at 30°C or 40°C, no seed could germinate. In other Ardisia species, the germination was always better at 30°C or 35°C than at the temperatures below 20°C [12-13]. This suggests that the seed germination of A. virens var. annamensis is more adapted to shade and warm habitats than other Ardisia species.
The effect of light on seed germination was relative to temperature. In lower temperature (10-15°C), light could significantly promote seed germination, but in higher temperature above 20°C, light had hardly any significant effect. In low temperature, the seeds buried in soil had low germination percentage, it is helpful for most of these seeds germinate in higher temperature and develop into seedlings. But in this study, so the germination was not in full darkness, because in the observation intervals, the seeds were exposed to a very short duration of weak indoor illumination which may be essential for germination for seeds of some plants. This may partially explain why most of the shrubs can grow in shady understory areas of dense evergreen broad-leaved forests. It has been found that seed germination become less dependent on light with increasing seed mass [14]. The 1000-seed weight of A. virens var. annamensis was over 300 grams and much larger than that of some typical small seed plant, such as Compositae, Gramineae, whose seed germination require light. But light did not significantly improve the seed germination of Ardisia crenata and A. virens at 10-35°C [12-13].
4.2. Effects of Desiccation and Storage on Seed Germination
The germination of desiccated seeds responded differently depending on the desiccation method [15-16]. The seed germination of A. virens var. annamensis declined when seed MC was dropped below 40% whether dried by the rapid dehydration or the slow dehydration method. When stored at 15°C and -20°C the seeds lost germinability more rapidly than at 4°C and 10°C. When seed MC was decreased to below 30%, all of the seeds lost their germinability. This indicates that the seeds of A. virens var. annamensis have a weak desiccation-tolerance. Therefore, the seeds should be classified as recalcitrant [16], which may be one of the reasons that this shrub prefers moist natural habitats. Some researches have shown that seeds of other Ardisia species, e.g., Ardisia crenata, Ardisia japonica, have similar seed germination characteristics [12,17,18], but the seeds of Ardisia crenata and its variety Ardisia crenata var. bicolor are more tolerant of desiccation than those of A. virens var. annamensis. The seeds of Ardisia crenata collected in late September or later months showed higher germination percentage, and germinated more rapidly after longer periods of storage at low temperature (approximately 5°C) [12]. This may partially explain why A. virens var. annamensis distributes in tropical and south subtropical zones, but A. crenata and A. crenata var. bicolor have wider distributions, not only in these two zones, but also in central subtropical zones in China. Other researchers have investigated the feasibility and methods of preservation for long-term seed storage in other plants by experimentally testing whether rapid desiccation can increase desiccation tolerance and storage longevity [15,17,19]. They also obtained some contradictory conclusions: For example Yao et al. found that for Ardisia elliptica, A. brunnescens and A. virens desiccation method and duration significantly affected cryotolerance, but rapid desiccation did not improve germination compared to slow desiccation and desiccation duration significantly affected germination percentage in the three species, especially after 48 h [19]. But in this study, rapidly desiccated seeds had somewhat more storage longevity than the slow desiccated seeds.
Desiccation of recalcitrant seeds is associated with damage and causes their germination percentage to decrease [12,16] and this is associated with an increase electrical conductivity of the leachate from the seed (Table 4). The longer was the duration of dehydration, the more serious was the damage. Slower drying means that more time is spent in the stage of dehydration where metabolic damage can occur [20]. Most of the freshly harvested A. crenata and Ardisia crenata var. bicolor seeds exhibited a low germination percentage, but could germinate after being placed for 30 d at 15°C, so the plant seeds had after-ripening period [12]. Whether A. virens var. annamensis seeds had the similar phenomenon needs further investigation, because in the present study, the seeds were stored in air-permeable plastic bags at 15°C storage room for 1 month after germination experiment, and the germination tests of freshly harvested seeds were not carried out. After-ripening in cold stratification or fluctuating-temperature stratification is found to accelerate seed germination of other plants [21]. But Yang et al. reported that the fresh seeds of A. crenata had the highest final germination [12]. The differences between theirs and ours in germination of fresh seeds may be caused by harvesting time and short-term storing methods before germination test.
Storage conditions affect seed longevity. Peng (2006) found the A. crenata seeds stored at room temperature under ventilation lost germinability, but those stored within humid sand at 5°C for 80 days reached 97% GP [22]. Yang et al. (2009) also found A. crenata var. crenata seeds stored in an air-ventilated room for 90 days fully lost germinability, but moist storage lengthened the seed life span. Collectively these data indicate that low temperatures and moist conditions are necessary for the seeds of A. crenata to survive in storage [12]. For orthodox seeds, dry and cold environment is necessary for seed storage, while recalcitrant seeds can not be dried before storage, and they should be stored in moist conditions, but moderate or low-temperatures are necessary to maintain seed longevity because a lower storage temperature can lower the metabolism intensity of seeds [16].
The fruits and seeds of A. virens var. annamensis remain on the maternal plants until late next spring, and the seeds remain alive in the late next spring and early summer. Therefore, we believe that the maternal individuals must be able to provide enough water to the undetached fruits to prevent dehydration damage to the seeds before the fruits drop. When the fully matured fruits drop from maternal shrubs in late spring and early summer, the warm and wet environment is suitable for seed germination and seedling establishment. Therefore, it seems that the fruit and seed maturation seasonality, and the characteristics of fruit retention on maternal plants in late spring are good adaptation mechanisms to avoid dehydration in the dry season for the seed survival of the species. To produce seedlings of this shrub in artificial cultivation, the seeds should be harvested in the winter and spring first, and then stored for about a month in a moist and cool environment to ensure the completion of probable after-ripening, then finally sowed in moist and aerated soil.
Seeds of Ardisia are considered recalcitrant and recalcitrant seeds are most commonly found in moist regions with an invariant climate [18]. In the native regions of A. virens var. annamensis in Yunnan and Southeast Asia, the wild populations have decreased because of forest fragmentation leading to the warming and drying of local climate [23] which may inhibit seed germination and seedling establishment of this shrub. For example, Xishuangbanna is the most abundant region for A. virens var. annamensis. In the recent two to three decades, the destruction of the tropical forests (cutting down the original natural forest and semi-natural forests and replanting rubber trees) in Xishuangbanna has increased fragmentation, and the local forest environment has become warmer and dryer [23], one of the evidences is that recent studies have shown the local annual rainfall and fogy days have been decreasing (Fan & Thomas, 2013).
5. Conclusion
The responses of seed germination to light, temperature and storage indicate that A. virens var. annamensis is well adapted to the tropical and subtropical moist environments in South and Southern-western China. From the results of field survey and characteristics of seed germination and storage, it is not hard to see that the climate change caused by human activities in this region may affect not only the fruit production of maternal trees, but also the seed germination and seedling establishment of this species. When artificially cultivating this plant, the timely seed collection, proper storage and timely sowing are helpful for improving seed germination and seedling emergence.
Acknowledgements
Authors wish to thank the Key Project of Science and Technology Innovation of Colleges and Universities in Guangdong Province (cxzd1132), the Special Funds of Talent Introduction of Colleges and Universities in Guangdong Province and the Knowledge Innovation Project of Chinese Academy of Sciences (KSCXZ-SW-117).
References
- Chen, C. and Pipoly J.J. (1996). Myrsinaceae. In: Wu, Z.Y. and Raven, P. H. (Eds.), Flora of China Vol.15, Science Press, Beijing, China. pp. 10–30.
- Kobayashi, H., DeMejía, E. (2005).The genus Ardisia: a novel source of health-promoting compounds and phytopharmaceuticals. Journal of Ethnopharmacology, 96: 347–354.
- Dat, N.T., Bae, K., Wamiru, A., McMahon, J.B., LeGrice, S.F., Bona, M., Beutler, J.A., Kim, Y.H. (2007). A dimeric lactone from Ardisia japonica with inhibitory activity for HIV-1 and HIV-2 ribonuclease H. The Journal of Natural Products, 70: 839-41.
- Li, J.X, Guan, K.Y., Yang H.S., Ma H., Li, H. Z. (2009). Plant resources of genus Ardisia in Yunnan. Guihaia, 29:236-241.
- Wang, J., Zheng, X.L., Chen Y.Z., Dai, H.F. (2013). Floristic Character and Geographical Distribution of Ardisia (Primulaceae) in Hainan Island. Journal of South China University of Tropical Agriculture, 4: 354-361,400.
- Luo, B.L., Zhou, R.,Wei, J.Q., Cheng, Z.Y., Li, H. (2010). The Research Advance of Cultivation on Ardisia. Hubei Agricultural Sciences, 49: 2909-2912.
- Wesley-Smith, J., Berjak, P., Pammenter, N. and Walters, C. T. (2014). Intracellular ice and cell survival in cryo-exposed emcryonic axes of recalcitrant seeds of Acer saccharinum: an ultrastructural study of factors affecting cell and ice structures. Annals of Botany, 113: 695-709.
- Finch-Savage, W. E. and Leucner-Metzger, G.. (2006). Seed dormancy and the control of germination. New Phytologist, 171: 501–523.
- ISTA (International Seed Testing Association). (1993). International rules for seed testing rules. Seed Science and Technology, 21: 79-287.
- Li, Y.H., Pei, S.J. and Xu, Z.F. (1996). List of Higher Plants in Xishuangbanna (2nd edition).Yunnan Nationalities Press, Kunming, China.
- Leite, I.T.A. and Takaki, M. (2001). Phytochrome and temperature control of seed germination in Muntingia calabura L. (Elaeocarpaceae). Brazilian Archives of Biology and Technology, 44: 297-302.
- Yang, Q. H., Ye W. H., Wang, Z. M., and Yin X. J. (2009). Seed germination physiology of Ardisia crenata var. bicolor. Seed Science and Technology, 2009, 37: 91–302.
- Yang, Q.H., Lan, Q.Y., Yang, H.S., Tan, Y.H., and Ye W.H. (2011). Germination, desiccation, storage and germination- accelerating pretreatment of Ardisia virens seeds. Seed Science and Technology, 39:327-337.
- Milberg, P., Andersson, L. and Thompson, K. (2000). Large-seeded species are less dependent on light for germination than small-seeded ones. Seed Science Research, 10: 99-104.
- Lan, Q.Y., Jiang, X.C., Song, S.Q., Lei,Y.B. and Yin, S.H. (2007). Changes in germinability and desiccation-sensitivity of recalcitrant Hopea hainanensis Merr. et Chun seeds during development. Seed Science and Technology, 35: 21-31.
- Hong, T. D. and Ellis, R. H. (1996). A protocol to determine seed storage behavior. In: Engels, J. M. and Toll, J (Eds.), IPGRI Technical Bulletin No.1, International Plant Genetics Resource Institute, Rome, Italy. pp. 1-51.
- Tezuka, T., Yokoyama, H., Tanaka, H., Shiozaki, S., & Oda, M. (2012). Seed and embryo germination in Ardisia crenata. Journal of Botany, DOI:10.1155/2012/679765.Lee, A. K., Slovin, J. P., Suh, J. K. (2012). Dehydration intolerant seeds of Ardisia species accumulate storage and stress proteins during development. Horticulture, Environment, and Biotechnology, 53: 530-538.
- Yao, X., Goodale, U.M., Li, Z.L., Lan, Q.Y. and Wang, X. F. (2014). Relative importance of seed drying rate, desiccation tolerance, and cryotolerance for the conservation of Ardisia elliptica, A. brunnescens and A. virens. Cryoletters, 35: 162-170.
- Ntuli, T.M., Pammenter, N.W., Berjak, P. (2013).Increasing the rate of drying reduces metabolic imbalance, lipid peroxidation and critical water content in radicles of garden pea. Biological Research, 46:121-130.
- Kaye, T.N., Kuykendall, K. (2001). Effects of scarification, cold stratification, and source population on germination of Lupinus sulphureus ssp. kincaidii. Seed Science and Technology, 29: 663–668.
- Peng L.B. (2006). A study on quality and germination of Ardisia crenata var. bicolor. Journal of West China Forestry Science, 35: 70-73.
- Zhao, A.L.‚ Chen, X.Y.‚ Zhang, X., Zhang, D. (2006). Effects of fragmentation of ever-green broad-leaved forests on genetic diversity of Ardisia crenata var. bicolor (Myrsinaceae). Biodiversity and Conservation, 15: 1339-1351.
- Fan, Z.X. and Thomas, A. (2013). Spatiotemporal variability of reference evapotranspiration and its contributing climatic factors in Yunnan Province, SW China, 1961–2004. Climatic Change, 116: 309-325.

 (Qihe Yang)
(Qihe Yang) 




