| 1. | ||
| 2. | ||
| 3. | ||
| 4. | ||
Hydroxyl Complexation in the System of Fe (III) - Fe (II) - Na(H)CIO4-H2O
M. M. Rahimova1, D. A. Davlatshoeva1, E. F. Fayzulloev1,
A. K. Ismatov2
1Department of Physical and Colloid Chemistry, Tajik National University, Dushanbe,
Republic of Tajikistan
2Scientific Research Institute, Tajik National University, Dushanbe, Republic of Tajikistan
Email address
 (M. M. Rahimova)
(M. M. Rahimova)  . (E. F. Fayzulloev)
. (E. F. Fayzulloev) Citation
M. M. Rahimova, D. A. Davlatshoeva, E. F. Fayzulloev, A. K. Ismatov. Hydroxyl Complexation in the System of Fe (III) - Fe (II) - Na(H)CIO4-H2O. American Journal of Chemistry and Application. Vol. 3, No. 3, 2016, pp. 13-18.
Abstract
By the classic method of Clarke Nicholas studied the oxidation potential of hydroxyl complexation of Fe (III) in the system: Fe (III) -Fe (II) - Na(H)CIO4-H2O, in the medium Na(H)CIO4. The experimental curves of the oxidation potential of one of the variables: pH, rS0 and PCR (negative logarithm of the concentration parameters) at constant rest. Analysis of the slope of the experimental curves of the method according to the theory, showed the formation of coordination compounds of the following: [Fe(OH)(H2O)5]2+, [Fe2(OH)2(H2O)10]4+, [Fe(OH)2(H2O)4]+ and [Fe(OH)3(H2O)3]0. The concentration of anions CIO4- affects iron hydrolysis, stability of the complexes, but not their makeup. By the method of successive approximations of experimental and Yusupov theoretical oxidizing function and using computer programs «Sigma Plot -10.0», «Excel» calculated stability constants are formed in the system of Fe (III) hydrolysis of complex forms.
Keywords
Iron, Hydroxyl Complexes, Oxidation Potential, Iron Hydrolysis, The Electrolytic Fund, Hydrolysis Constant
1. Introduction
At present great practical importance are the materials with nano-sized structure of the iron hydrolysis products and oxo compounds of different composition and stability, which depend on the ionic strength and pH. They are widely used as a new magnetic carriers magnetically sensors, application materials and sorbents, colloidal carriers for the delivery of active drugs in a magnetic field of medicinal auxiliaries, catalysts, sensors, pigments [1-4].
Hydroxide oxide and iron compounds are of great importance in microbiology. They are magnetotactic bacteria and iron-containing protein - ferritin are in a living cell "storage" of iron in the form of microcrystals of FeOOH. In addition, the hydroxide composites have physical and chemical properties of the crystallites are able to develop and evolve in a specific pattern to the transition in different crystallographic modifications and do not require large investments [5-7].
In the study of the formation of oxide and hydroxide of iron compounds in solutions to maintain a constant ionic strength, as a background usually are used different electrolytes. They are - differently affect the processes occurring in solutions. Some ions lead to a system of order and to the energy effect, while others weaken or even destroy a part of the hydrogen bonds. Saline environment can contribute to both increase and decrease central ion complexation, therefore, the composition, the stability of the region and the formation and existence of coordination compounds formed in the solutions depend on the background electrolyte [8-11].
In this paper we study the effect of the nitrate background on the processes of hydroxyl complexation of iron (II) and iron (III) by the oxidation potential [12, 13].
Joint analysis of the experimental curves of the oxidation potential of the system studied by the solution pH, inverse logarithm of the concentration of iron (III) (pC0) and iron (II) (pCR) revealed the composition and calculate the area of the existence of a sustainable manner hydroxyl complexes [14-16].
2. Experimental Part
Obtaining experimental data provides some preliminary work. This is a preparation and verification of platinum silver chloride and glass electrodes, glass electrode calibration, the synthesis of the starting materials and the standardization of their solutions. The nitrate, Fe (II) and Fe (III) were used as starting reactants, which are obtained by corresponding procedures [17]. Fe (III) concentration was determined by titration with 0.1 N solution of ascorbic acid in the presence variamina blue indicator and 0.1N solution of disodium salt of ethylenediaminetetraacetic acid (EDTA). The salicylic acid was used as a main indicator. The concentration of Fe (II) was determined by the presence of sodium diphenylamine sulfonate indicator with barometer method [17, 18].
For working solutions, equimolecular mixture of perchlorate solutions ferric iron, which was prepared in 1 M perchlorate acid solution, was used. To determine the total concentration of iron, Fe (III) was restored to Fe (II) in the Johnson gearbox which consists of a glass tube containing amalgamated zinc. The volume of reconstituted solutions of ferric salts should be from 100 to 150 ml. The free acid concentration of the solution should be about 2M, and the iron content is not more than 0.25 g of [19, 20]. Used salts were purified by filtration and then by saturated aqueous recrystallization. HClO4 concentration solutions prepared from fiksanal was determined by titrated NaOH solution.
For experimental curves of oxidizing potentialj from one of the concentration variables (pH, pCFe(III), pCFe(II)) is measured the EMF of electrochemical cells composed by a combination of the three electrodes without transfer. Used elements constitute as follows: to determine the pH - glass and silver chloride; for EMF measuring circuit - redox and silver chloride; to control the first and second readings of chains - silver chloride and glass.
Pt / test solution // NaClO4 (saturated) / KCl, AgCl (saturated) / Ag
Pt / test solution / glass / 0.1 mol / l HCl, AgCl / Ag
Ag / AgCl / 0.1 M HCl /glass/ test solution / NaClO4 (saturated) / KCl (saturated) / Ag
EMF measurements of described galvanic cells above were carried out on the ionomer EV - 74 with an accuracy of ± 1 mV. In the EMF measurement of corresponding electrochemical cells and the calibration of glass electrodes adhered to all the rules that are described in [1]. For glass electrodes were used mark ESP - ESP and 43/07 - 63/07. In all measurements the accuracy was ± 0.05 pH unit. The value of υ = 2.303RT / F values and silver chloride electrode potential at the necessary temperatures are taken from references [21, 22]. Studies were conducted under the following experimental conditions: [Fe(III)] = [Fe(II)] = 0.001 mol / L, T = 298.16 and 308.16 K and 0.1 ionic strength of the solution: 0.2; 0.25; 0.50; 1.00 and 3.00 mol / l.
3. Results and Discussion
According to the theory of the oxidation potential method determined the composition of the resulting of iron hydroxyl complexes, based on a joint analysis of the slope of the experimental curves of the oxidation potential of the pH of the solution, рС0 and рСr. The j oxidation potential decreases with dependence from increasing pH (Figure 1). This course of the dependence associated with a decrease of the Fe (III) concentration in the system, because of the iron consumed in the formation of hydroxo.
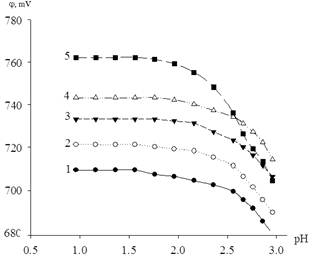
Figure 1. Dependence of the oxidation potential of the system Fe(III) - Fe(II) – Na(H)CIO4 - H2O from pH ata temperature of 298.16 K. The curves relate to solutions with CFe (III) = CFe (II) = 1×10-3 and ionic forces (mol/l) equal to 1 -0.10; 2 - 0.25; 3 - 0.50; 4 - 1.0; 5 - 3.0.
The curves of the figure obtained from the experimental values of the redox potential of the system and calculation using «Sigma Plot - 10.0» program. Draw the straight sections on the curves of this figure. According to the oxidation potential method theory [12-13] the following values of the angular coefficients: 0; ![]() and
and ![]() indicate the formation of hydroxyl forms of iron (III).
indicate the formation of hydroxyl forms of iron (III).
The amount of iron (III) atoms in the coordination compound is determined from the slope of the experimental dependence of the oxidation potential from the oxidized form of the metal concentration рСo (Figure 2). In the figure, you can highlight areas with linear slope equal ![]() and
and ![]() .
.
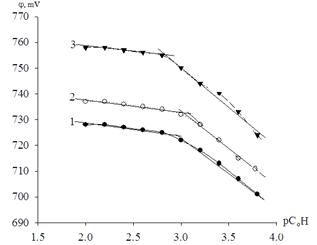
Figure 2. The dependence of the oxidation potential of the system Fe (III) - Fe (II) - Na(H)CIO4 - H2O from рСо at a temperature of 298.16 K. The curve is calculated by the program «Sigma Plot - 10.0», and points - the experimentally measured values, pH = 2.25. Curves refer, respectively, to the ionic forces: 1-0.5; 2-1.0; 3-3.0 mol / l.
An analysis of the first derivative of the general equation of the oxidation potential of the рСо shows:
![]() (1)
(1)
Value of q equal to 1 and 2 obtained at the slope of the experimental curve of dependence ofj from рС0, i.e. that according to the theory of the oxidation potential method corresponds to the formation of water-soluble mononuclear [Fe(OH)(H2O)5]2+, [Fe(OH)2(H2O)4]+ and dual hydroxyl complexes of iron [Fe2OH)2(H2O)10]4+.
To perform calculations using computer programs requires a chemical models of equilibria of studied oxidation - reduction system, which are based on the stoichiometric matrix (Table 1). It includes the slopes of the experimental curves, the interval of their location on the pH scale, the alleged structure of the complexes formed and their stability constants.
Table 1. The system of Fe(III) - Fe(II) - Na(H)CIO4 - H2O. Stoichiometric matrix slopes of experimental values of dependence of the oxidation potential from the concentration parameters at 298.16 K.
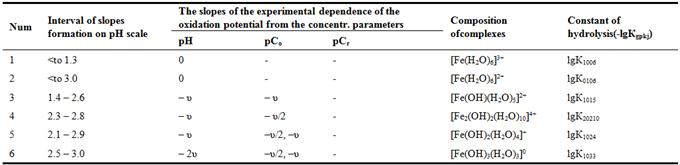
Table 2. The system of Fe (III) – Fe (II) – H2O – Na(H)CIО4. Reactions of the formation of hydroxyl complex compounds, their chemical model.
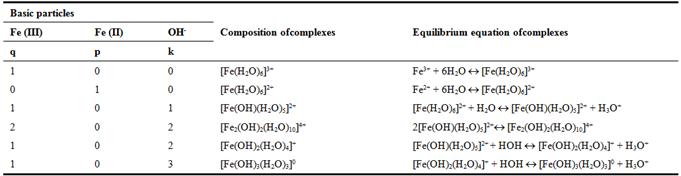
The chemical reactions model of formation of hydroxylcomplexes (Table 2) compiled using the data of Table 1for the studied system Fe (III) - Fe (II) - Na (H) CIO4 - H2O [5, 6]. Model includes a hypothetical composition of the forming hydroxo, the number of atoms Fe (III) and Fe (II) in the focal particles and hydroxyl groups and the reactions of complex formation forms [5-8].
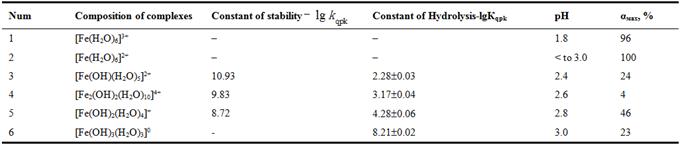
Constant calculation using an oxidizing function provides for the calculation of the equilibrium constants or hydrolysis. According to Fig. 1 and 2 as well as Table 3, for the system have been studied following hydrolysis forms of iron (III): [Fe(OH)(H2O)5]2+, [Fe2(OH)2(H2O)10]4+ and [Fe(OH)2(H2O)4]+. The iron (III)hydroxide - [Fe(OH)3(H2O)3]0 is precipitation at pH values above 3.2. For the intended composition of the compounds (Table 1) with an oxidizing function equation fragments were calculated approximate values of the hydrolysis constants and hydroxylcomplexes stability.
The Yusupov oxidizing function was used to calculate the ionic equilibrium in the studied system [13], to made chemical model and these computer programs. Previously, according to the known equation:
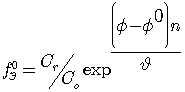 (2)
(2)
where: ![]() - experimentally measured value of oxidation potential,
- experimentally measured value of oxidation potential, ![]() o - the value of the standard oxidation potential, n- the number of electrons involved in the oxidation - reduction reaction, and υ = 2.303RT / F, calculated numerical values of experimental oxidative function provided С0 ≠ Сr, it built its dependence on pH.
o - the value of the standard oxidation potential, n- the number of electrons involved in the oxidation - reduction reaction, and υ = 2.303RT / F, calculated numerical values of experimental oxidative function provided С0 ≠ Сr, it built its dependence on pH.
Based on the general equation of the oxidation potential, taking into anticipated accounts of the complex obtained the equation of theoretical oxidizing function.
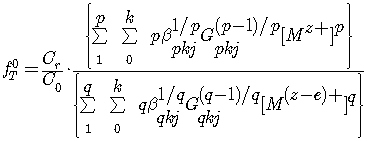 (3)
(3)
where: ![]() - the overall formation constant of the complex,
- the overall formation constant of the complex, ![]() - the concentration of polynuclear complex forms,
- the concentration of polynuclear complex forms, ![]() and
and ![]() - charges of complex particles;
- charges of complex particles; ![]() - the number of water molecules.
- the number of water molecules.
In view of the hydrolysis constant (![]() ) and the equilibrium concentration of the dimeric complex (
) and the equilibrium concentration of the dimeric complex (![]() ) final equation of theoretical oxidizing function takes the form:
) final equation of theoretical oxidizing function takes the form:
Table 3. The system of Fe (III) –Fe (II) – Na(H)ClО4 – H2O. Model parameters formation of hydroxyl complexes at 298.16 К, СFe(III) = СFe(II)= 1×10-3, ionic strength I = 0.50 моль/l.
![]() (4)
(4)
where: ![]() - theoretical oxidizing function; h = [H+] - hydrogen ion activity; K1015, K20210, K10210 and K1033 - constant values of the hydrolysis of the following hydroxo: [[Fe(OH)(H2O)5]2+, [Fe2(OH)2(H2O)10]4+,[Fe(OH)2(H2O)4]+ and [Fe(OH)3(H2O)3]0, respectively; G20210 - dimer equilibrium concentration of [Fe2(OH)2(H2O)10]4+.
- theoretical oxidizing function; h = [H+] - hydrogen ion activity; K1015, K20210, K10210 and K1033 - constant values of the hydrolysis of the following hydroxo: [[Fe(OH)(H2O)5]2+, [Fe2(OH)2(H2O)10]4+,[Fe(OH)2(H2O)4]+ and [Fe(OH)3(H2O)3]0, respectively; G20210 - dimer equilibrium concentration of [Fe2(OH)2(H2O)10]4+.
Using the method of successive approximations of the theoretical oxidative function with experimental after 8 - 10 iterations obtained the true values of the hydrolysis constants and of the hydroxyl complexes stability.
The numerical values, the degree of accumulation and the domain of existence of the iron hydrolysis constants constitute the model parameters of equilibriums and are presented in a separate Table 3.
Further, the degree of accumulation of the complexes were calculated according to the equations:
![]() (5)
(5)
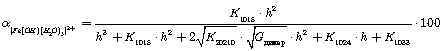 (6)
(6)
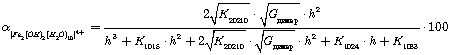 (7)
(7)
![]() (8)
(8)
![]() (9)
(9)
Using the oxidation potential values for given values of pH, defined experimental oxidative function, it built its dependence on pH (Figure 3).
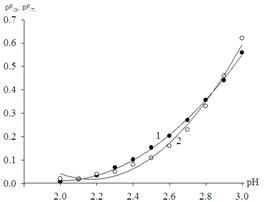
Figure 3. Dependence of the theoretical pf(т) and the experimental рf(э) oxidizing function of the pH of the solution for the system Fe (III) -Fe (II) - Na(H)ClO4 - H2O at 298.16 K and ionic strength of 0.50 mol / l.
1 - curve is drawn from the experimentally obtained points,
2 - theoretically calculated points.
The calculated values of degrees of the hydroxylcomplexes accumulation used to construct a distribution diagram (Figure 4).
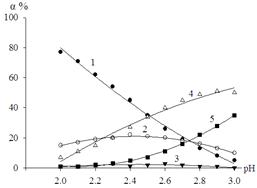
Figure 4. Hydroxo distribution diagram of Fe (III) in the system of Fe (III) - Fe (II) - Na (H) ClO4 - H2O at a temperature of 298.16 K, ionic strength of I = 0,50; СFe(III) = СFe(II)= 1×10-3 mol / l. 1 –[Fe(H2O)6]3+; 2 – [Fe(OH)(H2O)5]2+; 3 – [Fe2(OH)2(H2O)10]4+; 4 – [Fe(OH)2(H2O)4]+; 5 – [Fe(OH)3(H2O)3]0.
The maximum degree of accumulation - 46% corresponds to the complex composition of the [Fe(OH)2(H2O)4]+ at pH 2.8. For the complex [Fe(OH)(H2O)5]2+ this value falls to pH 2.4 and 24%. At pH 2.6 the maximum proportion of dimer [Fe2(OH)2(H2O)10]4+ is 4%.
Stability analysis Fe (III) hydroxo shows that they form the following series: [Fe(OH)(H2O)5]2+<[Fe2(OH)2(H2O)10]4+<Fe(OH)2(H2O)4]+<[Fe(OH)3(H2O)3]0.
By the method of Clarke Nicholas oxidative potential investigated processes of hydroxyl complexation of iron in the model system of Fe(III) - Fe(II) - Na(H)ClO4-H2O. It is shown that in a small range of solution pH (1.8-3.0) coexist several monomer [Fe(OH)(H2O5]2+, [Fe(OH)2(H2O)4]+, [Fe(OH)3(H2O)3]0 and iron (III) dimer hydroxo of composition [Fe2(OH)2(H2O)10]4+. Chemical model of equilibriums of the studied system compiled.
For the calculation of model parameters of complexes used the Yusupov oxidizing function. A small proportion of the dimeric form of [Fe2(OH)2 (H2O)10]4+ under studied concentration conditions at pH 2.6 indicates preferential formation process of mononuclear complex of [Fe(OH)3(H2O)3]0, that precipitates at increasing pH> 3.0 of solution. The coincidence of theoretical and experimental evidence of obtaining of reliable data and the possibility of using an oxidizing function and equilibrium modeling principle hydroxyl complexation oxidative functions to calculate the model parameters of coordination compounds using computer programs.
4. Conclusion
Using the Clarke Nicholas method of oxidative potential investigated the hydroxyl complexation processes of iron in the model system of Fe(III) – Fe(II) – Na(H)ClО4–Н2О. It shows that in a small range of solution pH (1.8-3.0) coexist several monomer [Fe(OH)(H2O)5]2+, [Fe(OH)2(H2O)4]+, [Fe(OH)3(H2O)3]0 and iron (III) hydroxo dimer of [Fe2(OH)2(H2O)10]4+ composition. It compiled the chemical equilibrium models of the studied system.
For the calculation of model parameters complexes used the Yusupov oxidizing function. A small proportion of the dimeric form of [Fe2(OH)2(H2O)10]4+ under studied concentration conditions at pH 2.6 indicates a preferential process of mononuclear complex of [Fe(OH)3(H2O)3]0 formation, that precipitates at increasing pH> 3.0 of solution. The coincidence of theoretical and experimental oxidative functions evidences about obtaining reliable data and the possibility of using an oxidizing function and modeling principle of hydroxyl complexation equilibrium to calculate the model parameters of coordination compounds using computer programs.
References