| 1. | ||
| 2. | ||
| 3. | ||
| 4. | ||
Interrelation of Thermodynamic Parameters and Structural Characteristics, with Halides of Groups 1 and 2 Elements as an Example
Nina V. Molchan1, Valery I. Fertikov2
1Research and Production Center "Pharmzashchita" FMBA of Russia (RPC "Pharmzashchita"), Khimki, Russia
2All-Russia Institute of Light Alloys (JSC "VILS"), Moscow, Russia
Email address
 (N.V. Molchan)
(N.V. Molchan)  (V. I. Fertikov)
(V. I. Fertikov) Citation
Nina V. Molchan, Valery I. Fertikov. Interrelation of Thermodynamic Parameters and Structural Characteristics, with Halides of Groups 1 and 2 Elements as an Example. American Journal of Chemistry and Application. Vol. 3, No. 5, 2016, pp. 28-32.
Abstract
The calculations of the concentration of electrons (Celektr mole/cm3) for simple substances and binary compounds on the basis of reference data on the density of matter in the condensed state are presented. A correlation dependence of the concentration of electrons with the thermodynamic characteristics of the halides of the elements 1 and 2 groups is revealed. The concentration of electron is proposed to use as a structural characteristic of the materials. The established relationship between the structural characteristics and thermodynamic parameters has allowed to theoretically calculate the thermodynamic parameters missing in contemporary references. The using of the coefficient of consolidation based the same reference data on the density of substances in the condensed state to compare the changes of molecular volumes during chemical processes.
Keywords
Concentration of Electrons, Density, Enthalpy, Entropy, Gibbs Energy, Halides, Structure
1. Introduction
Chemical transformations are accompanied by thermal processes and changes in the volume of substances reactive. The basic equation of thermodynamics in the following form
dU = TdS – pdV (1)
implies that the change in internal energy of the system is due to thermal energy when changing the volume dV. Thermal processes are considered in sufficient detail in many papers on chemical thermodynamics and thermochemistry both experimental and with the use of computational methods but there is actually no data available as for the change of volume in chemical reactions.
In the papers on the sulfides and oxides [1,2] it was found that the normalized value of the volume change named coefficient of consolidation (Kconsolidation, %) is correlated with the thermodynamic characteristics.
Since the volume of the molecule is formed by electrons, the term of concentration of electrons (Celectron, mole/cm3) [1-3] is introduced, which can be used as a structural material characteristics. Changing the structure of the material without changing the chemical composition is always accompanied by a change in the interaction of electrons in the material (phase transformations) [4,5].
Transformation of substances with the accompanying phenomena is the essence of the chemical form of motion, which is determined by the interaction of the electron shells of atoms and molecules.
The aim of this study is to investigate the possibility of using coefficient of consolidation as a characteristic to estimate the intensity of the interaction between heterogeneous atoms, and concentration of electrons determined in units of mole/cm3, as a value to estimate the material structure. The stated aim has been achieved by identifying dependencies of calculated electron density with the known thermodynamic properties of substances by the example of metal halides of groups 1 and 2.
2. Method of Calculation and Theoretical Basis
The computations below are based on the reference data on the density of substances in the condensed state (at temperatures below the melting point for each of substances) [6-8]. The value of the atomic volume (the volume of one mole of atoms of a chemical element, cm3/mole) characterizes the relative friableness of electron shells, and the reciprocal value of the atomic volume represents a concentration of nuclei of atoms per volume unit (mole/cm3):
![]() , (2)
, (2)
where CA is the concentration of nuclei, mole/cm3; d is the density of substance in the condensed state, g/cm3; M is the molar mass in g/mole.
According to the formulas presented earlier [1-3,9], the following values were calculated:
the concentration of electrons for the elements:
![]() , (3)
, (3)
the concentration of electrons for the compound of AaBb type:
![]() (4)
(4)
the coefficients of consolidation:
Kconsolidation =  , (5)
, (5)
where Celectron is the concentration of electrons per volume unit, mole/cm3; Z is the ordinal number of the element (ZA and ZB are the ordinal numbers of elements A and B respectively); a is the subscript of element A; b is the subscript of element B, Kconsolidation is the coefficient of consolidation, %; Vcomponent is the molar volume of the element in the condensed state, cm3/mole; Vproduct is the molar volume of the compound in a condensed state, cm3/mole.
The studies [1,3] show that the value of the atomic volume (cm3/mole) for the elemental substance in the condensed state (at a temperature below their melting point) represents the relative friableness of the electron shells, and as a result, the relative sizes of the atoms.
With regard to the structure of substance, the atomic mass is concentrated in its nucleus, and the volume is determined by its electron shell. The size of nucleus is different from the size of an atom in five degrees. The density of substance is determined by the atomic mass and closeness of atoms to each other.
All substances can be converted into a solid state from any state of aggregation simply by temperature decreasing. From a thermodynamic point of view, this transformation is characterized only by a decrease of the system entropy. There are no any chemical transformations at that. The density of two condensed states of liquid and solid substances of the same chemical composition varies in most cases within 10%.
In the course of chemical reactions deformations of the electron shells of the atom and the partial socialization of electrons from the reaction components occur. Consequently, the volume of the reaction product molecule may be equal to the sum of volumes of initial atoms, or it may be less or more than the sums of these volumes.
It seems hard to consider the radii of ions as such a linear geometric feature is almost not applicable to diatomic molecule due to the uncertainty of the volume fractions of a chemical element in the compound. In the first approximation the form of a diatomic molecule can be considered as katenoid-ellipsoid. It is the volume of the molecule that is physically measured and determined.
The reciprocal value of atomic volume for elemental substances (homoatomic compounds) is the concentration of atoms per volume unit. For heteroatomic compounds molar volume of condensed material can be determined by dividing the molecular weight of a chemical compound by its density. Dividing the sum of the stoichiometric indices of the atoms by the value of the molecular volume, we obtain the concentration of the atomic nuclei whatever the class of the atom. This approach allows us to consider the distribution of heterogeneous nuclei as the statistical distribution of the substance mass concentrators in the considered volume.
It is suggested to compare the volumes of the substances before the reaction (Vcomponent) and after the reaction of the compounds (Vproduct) calculated in cm3/mole for the substances in the condensed state on the basis of reference density data. It is suggested to call the resulting value normalized to the volume of the final product and expressed as a percentage the coefficient of consolidation (according to formula 5).
The interaction of atoms in chemical reactions depends on their ability to deform the electronic distribution of interacting components. Coefficient of consolidation allows to characterize any compound as a product obtained by either loosening (the value with the minus sign), or the consolidation of the electron shells in the interaction of its constituent elements.
Changing the structure of the material without changing its chemical composition is always accompanied by a change in the interaction of electrons in the substance. The value of the electron density (Celectron) characterizes the differences in electronic environment of the structure, which in its turn determines the processes that occur during the deforming effects and chemical reactions [10].
For the chemical compounds electron concentration is determined as the number of electrons per mole of the compound in relation to the volume of the solid compound (according to formula 4).
To understand the chemical changes occurring during the process, it is necessary to compare the individual characteristics of the substances before the interaction and after the process is completed. The substance during the transformation from the amorphous to the crystalline state becomes denser. Finely-grained structure of the alloy after annealing becomes coarse-grained, there is an increase in the density of the material at that [11,12].
3. Results and Discussion
Halides of alkali and alkaline earth metals are used in various chemical technologies. Technological processes result in changes in substances that can be estimated from a thermodynamic point of view.
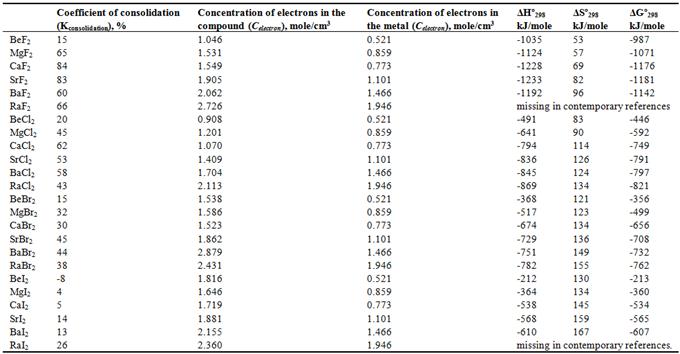
From the reference book [13] groups of halides of elements 1 and 2 groups have been selected, a comparison of the values of the enthalpy (ΔHº298, kJ/mole), entropy (ΔSº298, kJ/mole) and Gibbs energy change (ΔGº298, kJ/mole) has been carried out. The concentration of electrons of elemental halogen and parent metal forming halide have been calculated by formula (3), the concentration of electrons in the halide has been calculated by formula (4). The coefficients of consolidation (Kconsolidation) have calculated for all the selected halides according to formula (5). The calculation results are shown in Tables 1 and 2.
Validity of the calculations and comparative analysis based on these calculations confirmed that the graph of the concentration of the nuclei calculated by the formula (2) to the ordinal number of the element (see fig. 1) show periodic dependence [9].
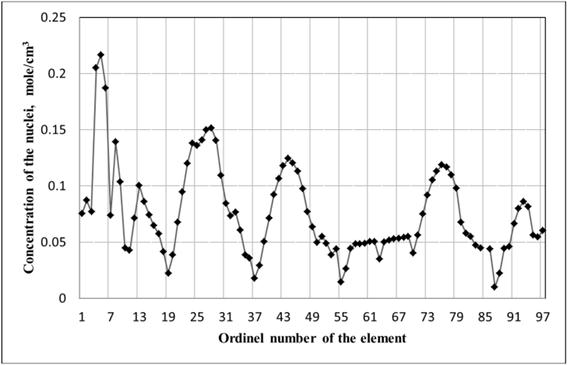
Fig. 1. Concentration of atomic nuclei for elements in condensed state (mole/cm3).
Halides of alkali metals are characterized by the fact that the elements of group 1 are the most reactive metals and halogens (group 17) are the most reactive of nonmetals. Between these groups under consideration there is group 18 of chemically inert elements.
The conсentration of electrons of the halogen element in the condensed state Celectron (mole/cm3) were: F 0.931, Cl 0.970, Br 1.358, I 2.062.
Further, using the Excel program the values obtained for a series of five similar compounds have been compared in pairs in order to identify the presence or absence of consistent pattern by calculating the correlation coefficient (R).
The critical value of the correlation coefficient for five pairs with a confidence coefficient of 0.95 is equal to 0.805. [14].
For alkali metal fluorides (Table 1) it has been revealed that there are correlation dependencies of coefficient of consolidation with entropy (R = 0.862), the electron densities in the compound with entropy (R = 0.863), the electron densities in the metal enthalpy (R = 0.899), the electron densities in the metal entropy (R = 0.936), the concentrations of electrons in a metal with a change in the Gibbs free energy (R = 0.883).
Table 1. The Coefficient of Consolidation, the Electron Concentration of Halides, the Concentration of Electrons in Metals of Group 1 and Thermodynamic Characteristics.

For alkali metal chlorides (Table 1) it has been revealed that there are correlation dependencies of coefficient of consolidation with enthalpy, entropy and Gibbs energy change (R = -0,973, -0,963 and -0,965 respectively), the concentration of electrons in the compound with an enthalpy, entropy and Gibbs energy change (R = -0.935, 0.975 and -0,920 respectively), and the concentrations of electrons in a metal with entropy (R = 0.957).
For bromides of alkali metals (Table 1) it has been revealed that there are correlation dependencies of coefficient of consolidation with entropy (R = 0.849) and the concentrations of electrons in a metal with entropy (R = 0.964). For the alkali metal iodides it has been revealed that there are correlation dependencies of coefficient of consolidation with enthalpy, entropy and Gibbs energy change (R = -0.908, 0.878 and -0,910, respectively) and the concentrations of electrons in a metal with enthalpy, entropy and Gibbs energy change (R = 0.850, 0.968 and 0,857 respectively).
It should be noted that all the coefficients of consolidation of fluorides, chlorides, bromides and alkali metal iodides are in the correlation relationship with the entropy (correlation coefficients are larger than the critical value).
Similar calculations have been made for halide elements of group 2 represented by metals that compared with Group 1 metals are less reactive. The calculation results are presented in Table 2.
Table 2. The Coefficient of Consolidation, the Electron Concentration of Halides, the Concentration of Electrons in Metals of Group 2 and Thermodynamic Characteristics.
For fluoride elements of group 2 correlation dependencies of coefficient of consolidation with enthalpy and Gibbs energy change have been revealed (R = -0.933 and -0,926 respectively). For chlorides of group 2 elements dependencies with correlation coefficient above critical have not been revealed.
For bromides of group 2 elements (Table 2) correlation dependencies with coefficient of consolidation with enthalpy and Gibbs energy change have been revealed (R = -0.883 and -0,880 respectively). For iodide elements of group 2 elements correlation dependencies of coefficient of consolidation with enthalpy, entropy and Gibbs energy change have been revealed (R = -0.932, 0.894 and -0,932 respectively).
With the difference in concentration of electrons in substances entering into a reaction, statistical concentration of electrons in the final product will be formed by redistribution of electrons from a higher concentration to a lower one, forming a new volume and as a result, a new density of the compound. Thus, the higher the electron density of the obtained compound, the higher the density of the product.
The relationship between Celectron values (mole/cm3) with the enthalpy of formation of a number of halides allows us to use the suggested value as an indicator characterizing the structure of the substance, the changes of which result in a change in the substance properties.
The established relationships allow us to calculate the value of the thermodynamic characteristics missing in the current references through the regression equation.
Thus, for instance, there are no available references of thermodynamic characteristics for RaF2 and RaI2. Based on the interrelation of coefficients of consolidation with enthalpy, entropy and Gibbs energy it has been calculated that ΔHº298 for RaI2 is (-790 kJ/mole), ΔGº298 = (-788 kJ/mole), ΔSº298 = 178 kJ/mole. For compound RaF2 thermodynamic quantities have been calculated respectively: ΔHº298 = (-1175 kJ/mole), ΔGº298 = (-1172 kJ/mole).
4. Conclusion
a It is suggested to use the reference data on the density of elemental substances in the condensed state to compare the changes of molecular volumes of substances.
b To estimate the degree of interaction between the initial components during the formation of compounds it is suggested to use the coefficient of consolidation.
c It is suggested to use the value of the concentration of electrons of an element or a compound, defined in terms of mole/cm3, as the structural characteristics of the material.
d A number of dependencies with a correlation coefficient above the critical for coefficients of consolidation and concentration of electrons with the thermodynamic characteristics have been revealed.
e The established relationship between the structural characteristics and thermodynamic parameters has allowed to theoretically calculate the thermodynamic parameters missing in contemporary references.
References