| 1. | ||
| 2. | ||
| 3. | ||
| 4. | ||
| 5. | ||
A Retrospective Study of Leukemia in Libyan Children
Hanan Abushwereb1, *, Salem Zaroug2, Salwa Othman1
1Pharmacology and Clinical Pharmacy Department, University of Tripoli, Faculty of Pharmacy, Tripoli, Libya
2Pediatric Oncology Department,Tripoli Medical Center, Tripoli, Libya
Email address
 (H. Abushwereb)
(H. Abushwereb) Citation
Hanan Abushwereb, Salem Zaroug, Salwa Othman. A Retrospective Study of Leukemia in Libyan Children. International Journal of Clinical Medicine Research. Vol. 3, No. 3, 2016, pp. 55-59.
Abstract
Leukemia is a cancer of the tissues that produce blood cells resulting in abnormal cells. Several forms of leukemia are reported, two of which are particularly common in children between ages of one month up to 16 years. These are known as Acute Lymphocytic Leukemia (ALL) and Acute Myelogenous Leukemia (AML). This work was conducted at Tripoli Medical Center (TMC), department of Pediatric Oncology to study child hood leukemia in diagnosed 50 Libyan leukemic children. This retrospective study was undertaken covering a period between 1997 and 2003, to describe the prevalence of most common types of leukemia and its relation to the family history, age and sex. It is also evaluated the rate of remission after chemotherapy. The results showed that ALL (73%) and AML (26%) are the most common types of leukemia occur in these children. Regardless gender type, the highest percentage was recorded between one month and 8 years of age. The incidence in males is higher than that in females, meantime no relation between incidence of leukemia and family history was observed. The highest cases of leukemia in these children were clinically reported in 1997 (24%) and in 2003 (22%). The most common symptoms reported among them were specifically fever, weight loss and fatigue. Chemotherapy protocols applied resulted in remission in 88% of cases while deaths was represented in 12% of all cases. In summary, further studies are needed to identify patients who are at high risk from failing of conventional therapeutic approach. Therefore, afforded the highest chance for a cure from childhood leukemia.
Keywords
Leukemia, Acute Lymphocytic Leukemia, Acute Myeloid Leukemia, Pediatric Oncology, Libyan Children
1. Introduction
Leukemia is a malignant disease of the bone marrow and blood. It is divided into two main categories: myelogenous or lymphocytic, each of which can be acute or chronic[1]. The terms myelogenous or lymphocytic denote the cell type involved. Acute leukemia is a rapidly progressing disease that results in the accumulation of immature, functionless cells in the marrow and blood. The marrow often can no longer produce enough normal red blood cells, white blood cells and platelets. Chronic leukemia progresses more slowly and allows greater numbers of more mature, functional cells to be made [2, 3].
In children, most common type of leukemias are acute. Approximately 80% of children with leukemia have ALL, while the rest have AML Incidence rates for all types of leukemia are higher among males than among females [4, 5]. The incidence of ALL among 1- to 4-year-old children is 9 times or more great than the rate for young adults ages 20-24 [6, 7, 8]. Children have a 20% to 25% chance of developing ALL or AML, if they have an identical twin that was diagnosed with the illness before age 6 [9]. Children at higher risk of developing ALL include those who have inherited certain genetic problems - such as Li-Fraumeni syndrome, Down’s syndrome, Kleinfelter syndrome, neurofibromatosis, ataxia telangectasia, or Fanconi's anemia – and children who are receiving medical drugs to suppress their immune systems after organ transplants [10]. Other risk factors include prenatal exposure to X-rays and prior radiation or chemotherapy for other types of cancer, usually within the first 8 years post treatment [11, 12].
Microscopically three subtypes of ALL can be identified: L1 (small uniform cells), L2 (large varied cells) and L3 (large varied cells with vacuoles). In most children, cells are designated as L1 and are related to B-lymphocytes, whereas in adults cells are defined as L2. B lymphocytic subtypes account for about 85 percent of ALL cases, while T- lymphocytic subtypes account for 15 percent of cases [13]. Abnormalities of chromosomes may also aid doctors in identifying a subtype of ALL [14]. For example, a change in chromosome 22, known as the Philadelphia chromosome occurs in a small percentage of children and a large percentage of adults with ALL [15, 16, 17].
Diagnosis of leukemia include the physical examination, taking the medical history, complete blood count (CBC) in addition to bone marrow aspiration, lymph node, and lumbar biopsies. The primary treatment of ALL is chemotherapy [18], which is complex and involves multiple drugs given in precise schedules and follow up over a period of two to three years. Once remission has occurred, maintenance chemotherapy is usually used to keep the child in remission. Maintenance chemotherapy is given in cycles over a period of 2 to 3 years. Intensive leukemia chemotherapy has certain side effects, including hair loss, nausea and vomiting in the short term, and potential health problems down the line. As a result, the child should be regularly monitored for those side effects [19]. Sometimes a bone marrow transplant may be necessary in addition to, or instead of, chemotherapy. With the proper treatment, most childhood leukemias have very high remission rates. Some forms of childhood leukemia have a remission rate of up to 90% and the majority of children can be succefuly cured of the disease [20, 21].
The aim of this study is to determine the prevalence of most common types of leukemia in Libyan children, as well as its relation to age, sex, and the effectiveness of chemotherapy protocols applied.
2. Methodology
Fifty cases of leukemia were selected from Tripoli Medical Center during the period of 1997 to 2003. The following points were documented according to (Stanford Children’s Health Network) [22]:
Ÿ Patient's general information (age, sex, birth place);
Ÿ Family’s and patient’s medical history.
Ÿ General examination of the patient’s symptoms with CVS, CNS, GIT and chest examination.
Ÿ Types of Leukemia as classified in the hospital record.
Ÿ Laboratory investigation performed include blood cell counts, liver and kidney function assessment, spinal fluid analysis, bone marrow biopsy for histopathologic examinations.
Ÿ Drugs used in treatment of leukemic patients, their dosages, route of administration and death cases post-treatment were also recorded.
3. Results
Fifty cases of leukemia between ages of 1 month and 16 years of age were studied. Acute Lymphocytic Leukemia (ALL) was the most common type of leukemia reported in those children and represented 74% of the cases; Acute Myelogenous Leukemia (AML) is the other form of leukemia and was recorded in 26% of patients. No relation with the family history was noticed in all cases. However, the incidence in males is higher (54%) than that in females (46%) (Figure 1). the annual total cases recorded between 1997 and 2003 showed in Figure 2 with highest rate (24%) in year 1997, followed by gradual decrease until the year 2001 (6%) then the rate increased again up to 14% in 2002 reaching 22% in the year 2003. Leukemia is more commonly developed in children with ages between 1 month and up to 8 years (44%) of age with the highest percentage occurring between ages 6-8 years Figure 3. The different subtypes of ALL are shown in Figure 4; ALL-L1 revealed that the predominant subtype occurring in 88% of the children, followed by ALL-L2 and then ALL-L3.
The most common symptoms reported in these patients were fever and weight loss Figure 5. Table 1 showed the most common drugs used for chemotherapy, their recommended dosages and route of administration. The protocol was designed according to the international policy of drug treatment depending on the cases (age, gender, disease severity and subtype). Chemotherapy induced remission in 88% of patients while death occurred in 12% of the treated cases Figure 6.
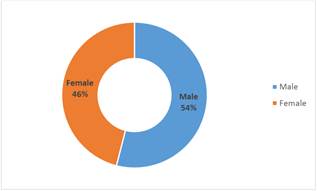
Figure 1. The gender percentage of leukemic children recorded in the Oncology ward of TMC (1997-2003).
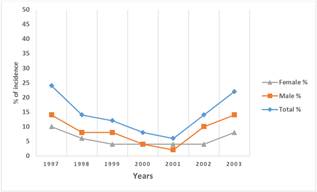
Figure 2. Annual cases of leukemic children clinically reported between year 1997 and 2003 at the TMC, Tripoli, Libya.
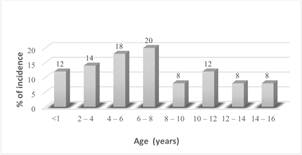
Figure 3. Age-dependent incidence in leukemic Libyan children in TMC.
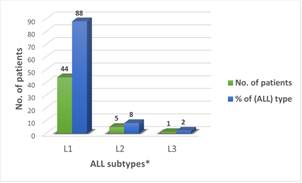
* L1, L2, L3 are the investigated ALL subtypes of leukemia.
Figure 4. The ALL subtypes recorded among 50 patients under study.
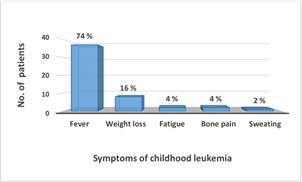
Figure 5. The common symptoms of childhood leukemia recorded among 50 patients under study.
*Percentage at the top of column represent percentage of the cases related to 50 patients (100%).
Table 1. Drugs, dosages and routes used in leukemia treatment protocol.
| Drug | Dose |
| Prednisone (Delatasone) | 40 mg |
| Dexamethasone (Dexone) | 6 – 8 mg |
| Vincristine (Vincasar) | 1.5 mg, i.v. |
| Asparaginase (Elspar) | 6000 – 25000 units, i.m. |
| Daunorubicin (Cerubidine) | 25 mg, i.v. |
| Methotrexate (Folex) | 20 – 8000 mg, i.v., i.m. |
| Cyclophosphamide | 300 – 1000 mg, i.v. |
| Cytarabine (Cytosar-U) | 300 – 3000 mg, i.v. |
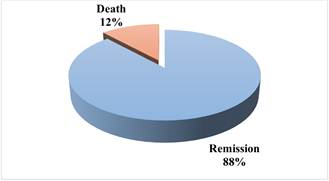
Figure 6. The final outcomes of leukemia therapy applied to 50 leukemic children (1997-2003).
4. Discussion
Leukemia is the major and common cancer causing more death than any other form of cancer in children. About 32 percent of cancers in children aged 0-14 years are leukemia [23]. Approximately 60% of children with leukemia have ALL, and about 38% have AML while, ALL is the most common malignancy diagnosed in children under 19 years of age, with a peak incidence in children aged 2-5 years. [24, 25]. This retrospective clinical study showed that ALL is the most common form (74%) in the 50 Libyan children involved. It occurred in younger children; aged between one month to 8 years, with a peak incidence recorded between ages 6 and 8 years. This might be referred to the small number of cases involved in this study. The results also indicated that there was no relation between ALL and family history. This means that gene mutations and chromosome abnormalities in cells occur incidentally and are not inherited from a parent. With the exception of prenatal exposure to X-rays and specific genetic syndromes, such as Down's syndrome, little is known about the causes of and risk factors for childhood ALL [11, 12, 22]. Leukemia is considered as a result of an interaction between hereditary genetic predisposition and environmental influences [26]. Many environmental factors (e.g. exposure to ionizing radiation, electromagnetic fields and pesticides and herbicides, parental use of alcohol, contraceptives and tobacco) have been investigated as potential risk factors, but none has been definitively shown to cause lymphoblastic leukemia [27, 28].
Generally, the ALL in children occurs slightly more often in boys than in girls. This has been related to differences in T -cells between males and females [21]. Concerning ALL, the L1 subtype was the most common in the investigated leukemic children, whereas L2 commonly occurs in adults. ALL-L3 rarely occurs. In the selected Libyan children, the annual cases of ALL patients were as high as 24% in 1997, decreased gradually until 2001 (6%), then increased again in the next two years. This may be due to poor diagnostic methods, and unsuitability of drugs or single-agent chemotherapy. In the recent years, the investigation methods have established the relationship between administered dosage and plasma (or tissue) concentration to reach the therapeutic goal with little toxicity. The uses of combined chemotherapy and radiation also helped a lot [29]. The increased incidence of leukemia during the last two years of this study (2002-2003) may result from the increased use of chemicals, chemical contamination of groundwater, X- ray radiation and other life threatening factors [30, 31].
Children with ALL develop symptoms related to infiltration of blasts in the bone marrow, lymphoid system, and extramedullary sites, such as the central nervous system [32]. Common symptoms include fever, fatigue, pallor, and weight loss. Infiltration of blast cells in the marrow cavity and periosteum often lead to bone pain and disruption of normal hematopoiesis [33]. Although the same symptoms have been recorded in the children, their percentage was lower. The differences in the results may be related to the small number enrolled in the study and its retrospective nature. In about 5% of children with ALL, spread of leukemia to the brain causes headaches, seizures, balance problems, or abnormal vision [6, 21, 33].
The primary treatment of ALL is chemotherapy. The goals of induction therapy are to attain remission, defined as the absence of evidence of leukemia, which includes the absence of CNS or testicular disease, and to have bone marrow examination showing normal cellularity with fewer than 5% lymphoblasts. The 3-drug combination of vincristine, a glucocorticoid, and L-asparaginase successfully induces remission in 95% of children with ALL [34, 35]. Improvements in diagnosis and advances in treatment have produced cure rates that may exceed 80% [2]. Treatment of childhood ALL in this study have led to long-term, event-free survival rates of about 88%. Given immediately after remission induction, consolidation therapy is particularly important in high-risk ALL, such as T-cell ALL and the infant form of ALL, in addition to patients with CNS disease. Maintenance chemotherapy usually includes daily administration of 6-mercaptopurine (6MP) in addition to intermittent methotrexate. Duration of therapy differs among centers and protocols, but, on average, therapy lasts 2-3 years. Generally, boys are treated for 3 years and girls 2 years, secondary to the recognition that boys are more likely to relapse [36].
Because of the relatively high rates of disease-free survival in ALL, hematopoietic stem cell transplantation is reserved for patients who fail to respond to induction therapy, those who relapse during treatment, or those who relapse within 1 year following completion of chemotherapy
The future of ALL therapy involves gene-expression profiling and immunophenotyping to classify children into various risk groups that will influence treatment decisions [37]. Patients between the ages of 2 and 10 years tend to have a more favorable prognosis. Infant ALL carries a high risk of early relapse despite intensive chemotherapy. These infants commonly have a subtype of ALL in which a specific gene is modified. This subtype of ALL is very hard to treat successfully and only a minority of infants with this subtype of ALL survives with current therapy. Other subtypes of ALL in which different genes are modified occur more commonly in older children and have a much more favorable outcome [38, 39, 40]. However, recent genome-wide profiling of germline and leukemic cell DNA has identified novel submicroscopic structural genetic alterations and sequence mutations that contribute to leukemogenesis and may define new subtype. Innovative approaches are needed to further improve survivals while reducing adverse effects and influence responsiveness to treatment that may provide novel prognostic markers and therapeutic targets [41].
5. Conclusion
Cancer remains the leading cause of death in children and the term treatment toxicities continue to affect the majority of children with cancer. Thus, the incorporation of targeted new agents into current chemotherapy regimens offers the prospect of potentially more effective and less toxic treatment for children.
References