| 1. | ||
| 2. | ||
| 2.1. | ||
| 2.2. | ||
| 3. | ||
| 4. | ||
Neuropsychological, Post-Stroke Improvement with a New Combination of Approved Substances: A Case Series Report
Felician Stancioiu*, Daniela Catanas
The Bio-Forum Foundation, Bucharest, Romania
Email address
 (F. Stancioiu)
(F. Stancioiu) Citation
Felician Stancioiu, Daniela Catanas. Neuropsychological, Post-Stroke Improvement with a New Combination of Approved Substances: A Case Series Report. International Journal of Clinical Medicine Research. Vol. 3, No. 4, 2016, pp. 64-71.
Abstract
A new combination of substances, already in use individually for treating various human pathologies, was administered intravenously to 11 neurologically normal patients and 3 patients with stroke or transient ischemic attack which occurred at least 1 week prior to treatment. A total of 22 such infusions were given beginning with October 2014 with good results and minor side effects (restless first night after infusion). The combination was individually adjusted and consisted of: deproteinated calf serum, dimethylsulfoxide, oxytocin, glutathion, dopamine, vitamins B (group) and C; it had positive and notable effects both in neurologically normal and in patients with neurological pathology (post-stroke).
Keywords
Stroke, Post-Stroke, Regenerative, Neuropsychological, Chronic Stroke Treatment, Regenerative Medicine
1. Introduction
We are witnessing exciting times in the field of neurology, so much so that during our lifetime two major tenets of neuroscience were overturned: in 1999 adult neurogenesis was evidentiated (neuron precursors migrating and maturing into neocortex from periventricular areas) in certain areas of the primate brain [1] and in 2015 was shown a direct connection between the immune system and the brain with the mapping of the brain lymphatic system [2]. More exciting news are expected to follow on the molecular level, as we recently found out [3] from laboratory experiments that interferon-γ modulates neuronal connectivity and the activity of brain areas involved in social interaction, and this adds to the empirical observations made by clinicians and patients during treatment of viral hepatitis that interferon-α has important behavioral effects besides its immune actions.
There are exciting news on the therapeutic side also, especially with stem cell-based therapies, with very promising results obtained after injecting embryonic and/or adult neural stem cells directly in the spine or brain of stroke patients [4-6]. However there are important limitations on their use, starting with ethical concerns, immune reactions and uncontrolled proliferation, and culminating with the belief among specialists that the best neural stem cells which can be used in stroke recovery are those already present in the patient’s own brain.
In this line of thinking we have tried to use existing approved substances which areknown from clinical practice and trials to have a positive effect on cell structure and function (deproteinated veal serum, dimethylsulfoxide, vitamins, etc.); they have pleiotropic actions via known pathways and molecules (brain-derived neurotrophic factor – BDNF, transforming growth factor beta – TGF β, stromal cell-derived factor 1α/CXC chemokine receptor 4, PI3K and p38 MAPK signaling pathways), as well as ones which need to be studied [7-9]. To these we have selectively added oxytocin, dopamine and glutathione as we aimed for a therapeutic combination with the role of stimulating endogenous neural cells precursors to differentiate, migrate and replace damaged neurons and neural networks, translated in recovery of neurological function, and we are reporting good results here.
2. Materials, Patients and Results
There were 22 intravenous treatments administered to a total of 14 patients between October 2014 and July 2016, in an ambulatory setting (day clinic) or at patient’s home, with continuous monitoring of the vital signs and patient status by a physician during infusions, followed by a short evaluation post-treatment.
The base solution was normal saline 250 or 500 ml; the dosage of substances used in this combination was slightly different for each patient and consisted of deproteinated veal serum (Actovegin) 4-15 mg/kg, dimethylsulfoxide (DMSO) aprox. 0.1 ml/kg, vitamins B1 100 – 300 mg, B6 250 – 750 mg, vit C 750 – 2000 mg, calcium gluconate 300-950 mg, oxytocin 10-15 UI, and in selected patients drotaverin, dopamine, glutathion, and a more complex combination of vitamins (Soluvit: Vitamin B1 - 2.5 mg, Vit B2 - 3.6 mg, Nicotinamide - 40 mg, Vitamin B6 - 4 mg, Pantotenic acid - 15 mg, Vitamin C - 100 mg, Biotin - 60 µg, Folic acid - 0.4 mg, Vitamin B12 - 5 µg).
Actovegin is a deproteinized hemoderivative produced from calf blood by ultrafiltration which contains more than 200 low–molecular weight compounds of up to 5,000 Da with pleiotropic biological actions [9]. A more detailed composition of Actovegin was given by Gulevsky et al in the 2011 study on cord blood and Actovegin in ulcer healing [10]. By using various analytical methods (chromatography, electrolyte analyzer, EIA immunoessays, folin phenol, glucose oxidase, etc.) he obtained the following data: Total peptides: 0.5 ± 0.03 mg/mL; Estradiol: 21.2 ± 2.0 nmol/L; Testosterone: 0.7 ± 0.6 nmol/L; Cortisol: 98.4 ± 6.0 nmol/L; Potassium: 67.8 ± 3.0 mmol/L; Sodium: 298.0 ± 10.0 mmol/L; Calcium: 0.7 ± 0.2 mmol/L; Glucose: 7.1 ± 0.3 mmol/L; Total glycoproteins: 4.9 ± 0.3 mmol/L; Hyaluronic acid: 0.4 ± 0.2 µg/m; Sialic acids: 2.9 ± 0.1 nmol/L.
The biological actions of the veal serum are numerous: it enhances oxygen absorption, oxygen utilization, ATP synthesis and cellular energy metabolism by mitochondria, exerts insulin-like activity (stimulation of glucose transport, pyruvate dehydrogenase, and glucose oxidation) possibly via a supplementation of inositol-phosphate-oligosaccharides (IPO) [11-14].
Even though multiple studies showed that Actovegin significantly improves post-stroke recovery compared to placebo, we surmised that results can be improved by adding other components. One direction for improvement can be inferred from the same study with Actovegin vs. cord blood on ulcer healing done by Gulevsky [10]. He compared the inflammatory aspect of the wound as measured with TBARS essay and alkaline phosphatase levels, and showed the cord blood to be significantly better; it also had better recovery of tissue microcirculation and decline in leukocyte infiltration than calf serum. Taken together this can be interpreted that the beneficial effects of Actovegin can be further improved by adding antioxidants and anti-inflammatory substances as well as other trophic factors for regenerating the nervous tissue and glia - all cells which support neurons’ structural integrity and activity. Besides vitamins B and C, oxytocin, DMSO and glutathione were thus chosen for their known antioxidant and anti-inflammatory properties, and other biological actions which will be discussed in the respective section:
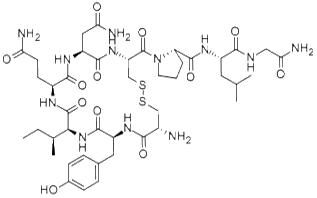
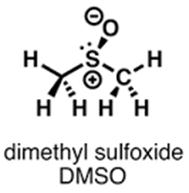
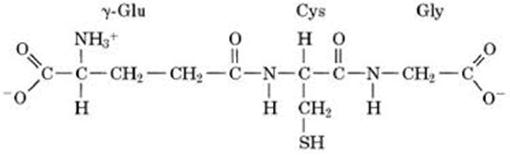
Figure 1. Oxytocin, DMSO and glutathion: chemical structure and active moieties.
In order to evaluate its potential benefits, the above combination was administered first to 11 neurologically normal patients including the 2 authors and afterward to two patients with stroke and one with transient ischemic attack; results are summarized below:
2.1. Neurologically Normal Patients
Beginning in October 2014 the combination was administered with individual adjustments to 11 neurologically normal patients including the 2 authors (7 females, 4 males, age range 36 – 72).
Clinical examination in these patients was within normal limits, with no acute distress reported and no negative findings; blood pressure range was from low normal (105/60) to stage II essential hypertension (165/100), pulse 60-88 bpm, BMI 17.8 – 32.8 (weight 52 – 104 kg); the anthropometric data is summarized in Table 1.
Table 1. Anthropometric data of neurologically normal infused patients.
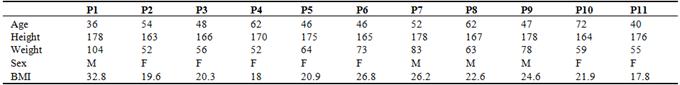
P1 – P11: patients with respective data
As these 11 patients had no manifest neurological pathology, they only presented complaints suggestive of sub-clinical impairments. Based on the clinical signs and symptoms noted, and upon further minimally invasive clinical examination and testing, we established criteria grouped in three categories:
a. central nervous function imbalance or peripheral neuropathy:
• insomnia or sleep disturbances (difficulty falling asleep, intermittent sleep, nightmares, early awakening, sleep duration of 5 hours or less, waking tired in the morning)
• difficulty in focus, concentration, short-term memory
• paresthesia (tingling/numbness on fingers/arms/leg)
• intense food cravings with nighttime food intake (bulimia nervosa) and obesity
• excessive perspiration of palms (overactive sympathetic tonus)
• complaints of low energy,
b. cardiovascular signs:
• palpitations with various frequency, from 1/week to a few daily
• elevated cholesterol, C-reactive protein/fibrinogen/erythrocyte sedimentation rate
• abnormal platelet distribution volume
c. other signs, symptoms and personal history:
• skin hyperpigmentation (facial, suborbital);
• thyroid abnormalities (elevated ATPO - thyroid peroxydase antibody)
• healing fracture (humerus), colon carcinoma, chronic viral infection, etc.
Patients were infused if they presented with at least three signs and symptoms; the number of infusions given was based on the presence of signs and symptoms from one or more categories:
• only one infusion if there were only signs and symptoms from the neuropsychological category.
• multiple infusions if there were also non-neurological signs and symptoms (cardiovascular, other).
Most of the patients (8) received 1 such iv infusion; in three patients were administered 5, 3 and respectively 2 such intravenous combinations; the interval of administration in these 3 patients was 5-7 days (Figure 2).
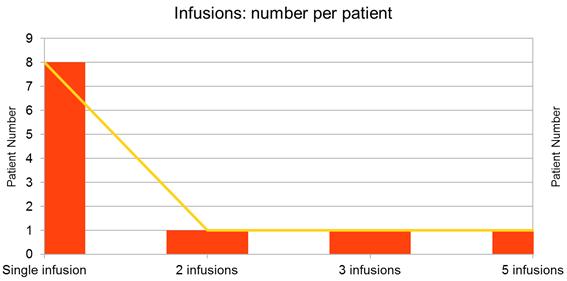
Figure 2. Number of infusions and patients.
During the infusions there were no significant changes in blood pressure or pulse (Table 2. Clinical data) except one patient (P7) who had a transient blood pressure drop from 135/80 to 100/60 with no modification of pulse; this patient felt no discomfort and the blood pressure returned to initial values at the end of infusion.
Table 2. Clinical data.
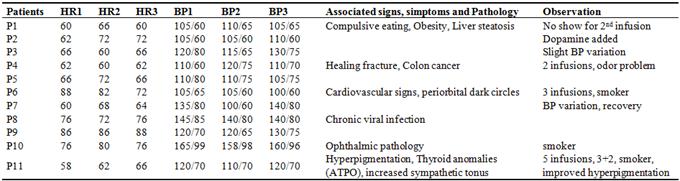
HR1, HR2, HR3– heart rate at beginning, during and at end of iv infusion; BP 1, BP 2, BP 3 – blood pressure initially, during and at the end of iv administration; P1 – P11: patients
The pervasive and most noticeable effect during the intravenous administration of this combination, both as reported by patients and observed by medical staff, was a decrease of the psychological tension and perceived stress without affecting focus and attention, and the onset of a peaceful feeling combining calmness and serenity (most likely an oxytocin-related effect), so that at the end of the infusion and final examination, the patient was able to ambulate normally and drive uneventfully.
Other improvements in central nervous system functioning were noticed after the first administration and consisted in improved focus/concentration, less fidgeting, more calmness, better sleep, more energy/less fatigue; those effects were maintained for at least one week after the infusion. Hyperpigmentation was greatly and promptly improved in two patients, one with periorbital dark lines and another with multiple facial spots probably due to thyroid (tyrosinase) dysfunction (both smokers).
Dopamine was administered in one patient; she felt uncomfortable during administration, reporting feeling slightly dizzy and feeling strong heartbeats; those subsided when dopamine was stopped and the second bag of 250 ml normal saline solution supplemented with drotaverin was administered.
There were no allergic reactions observed nor experienced after repeated infusions; one patient had complaints of very unpleasant garlic-like odor (DMSO - related) after the second perfusion and refused the third infusion. No other discomforts or side effects were noted.
2.2. Patients with Neurological Pathology
Besides the 11 neurological normal patients, there were 3 patients with neurological pathology to whom this combination was given between November 2015 and July 2016.
Stroke patient 1. - administered 2 infusions in November/December 2015
71 year old male, 168 cm, former smoker, history of obesity (abdominal), hypertension, high cholesterol and hyperglicemia, had a stroke about 10 years before, which left him with right side hemiparesis and mild speech impairment. While on blood pressure medication, in October 2015 had a second stroke which left him with no vision in left eye, left facial paresis, dysphagia for solids, severe speech impairment, irritability, mood swings and frequent depressive manifestations which together with dysphagia made him lose weight to the point of emaciation (loss of subcutaneous fat and loss of muscle in a catabolic state). The patient was cared for at home by his family and could not be weighted.
He was given a combination consisting of Actovegin, DMSO, oxytocin, vitamins B1, B6, B12 and C, drotaverin in 250 ml normal saline as single intravenous infusion, which was well tolerated with no complaints during and a few hours after the infusion, when the patient felt more calm and serene. Blood pressure before infusion was 160/90 and 140/80 after infusion, pulse varied between 72 – 86 beats per minute.
Family reported that during the first night the patient felt agitated and could not sleep, but next day he reported "seeing double" (left eyesight recovered with uncoordinated ocular muscles and diplopia). The patient also reported better control of left side of face and manifested slight improvement in speech, but most importantly had great improvement of dysphagia and was able to eat solid food (first pureed then small pieces of vegetables and meat). Physical therapy was started with the patient being able to stand first on the edge of the bed and later on in front of the window.
After 3 days post-infusion, a series of intravenous administrations of Cerebrolysin were started, but there was no further improvement in the patient status except for weight gain.
A few weeks after the first infusion, a second infusion with the same combination was administered, and after another agitated night, an improvement was noticed in his motor abilities and coordination, with the patient being able to turn by himself in bed (from laying on the back to turning on the right side).
The patient’s family has refused further infusions and he has continued physical therapy and more series of Cerebrolysin infusions, but no further improvement in patient status was observed.
Stroke patient two – one infusion in December 2015
58 year old female, 168 cm, 73 kg, slightly overweight (BMI around 27) with increased abdominal circumference, normal cholesterol with liver function tests (AST/ALT) suggestive of non-alcoholic fatty liver disease, severe insomnia and moderate anxiety for which she was treated with benzodiazepines prior to the stroke, hypertension treated with ACE inhibitor and betablocker but not well controlled, with frequent episodes of high values (180/100). During one such episode she had severe disarthria and paresthesias on right arm, for which she was admitted and had a negative CT scan for hemorrhage, but with multiple demyelinating areas. Calcium blocker was added for hypertension, blood pressure was successfully lowered to around 100-120 mm Hg systolic and 60 diastolic, but the patient had a worsening of status, with complete loss of motor and sensory function of right arm and moderate to severe motor dysfunction of right leg while in hospital (unable to ambulate unaided). She was discharged home in stable condition for physical therapy, and 1 week after the stroke she received at home the combination of Actovegin, DMSO, oxytocin, vitamins B1, B6 and C, which she tolerated well, with no modifications of blood pressure or pulse.
During and after the infusion she reported feeling more calm and less stressed, but during the night she did not sleep well; she was agitated and restless, mostly because of right shoulder and arm discomfort. However she reported regaining some sensory function in the right arm, first paresthesias during the night and afterward there were slight improvements in motor function of the right arm as she began moving fingers and forearm and in a few days she was able to hold a cup in her right hand and also walk better and for longer. A second infusion was not administered to the patient due to logistical issues; afterward the patient started treatment in a recuperation clinic and had significant but incomplete recovery of motor function six months after the stroke.
Third patient: transient ischemic attack with persistent neurological problems: one infusion, July 2016
39 year old female, 173 cm, 64 kg, had a transient ischemic attack 3 months before the initial evaluation and infusion. There were no MRI changes, and she recovered with no motor or sensory deficits except for absent patellar reflexes and absent Babinsky bilaterally. Has had vertigo, headaches, anxiety, paresthesias on both arms and for the prior 2 weeks had tachicardia and palpitations and frequent precordial pains occuring spontaneously and when upset and which sometimes felt like a claw. Patient was taking benzodiazepine for sleep and anxiety symptoms.
Physical examination was unremarkable except for absent tendinous reflexes bilaterally and absent Babinski. One-lead EKG showed no abnormalities of rhythm, Q wave or ST segment. Cardiac workup showed normal cholesterol, C reactive protein, troponin, CKMB, with normal liver and kidney function. At patient’s initiative, a serum mercury level was measured and it was 6 times over normal limit; the patient presented for infusion with a 3 kg weight loss (61 kg) and reported throwing up a few times the respective morning; however she was in no acute distress.
One intravenous infusion was administered consisting of 250 ml normal saline with Actovegin, DMSO, oxytocin, vitamins B1, B6 and C, and calcium gluconate. EKG performed during and after the infusion showed normal sinus rhythm with normal Qtc, no pathological Q wave or ST segment modifications, just a repolarization anomaly in precordial leads (slanted S wave). Treatment was well tolerated by the patient, who reported no discomfort and a feeling of calmness; she left the clinic in good status. However the patient was very bothered by the garlic-like odor which manifested from day 2 until day 5 post-infusion and even though the patient reported feeling better, not needing benzodiazepine, and having less frequent and less intense precordial pains (one week post infusion patient reported not having precordial days for two consecutive days), she decided to postpone the next infusion treatment for 1 month in favor of oral medication.
3. Discussion
There seem to be three major therapeutic directions for re-establishing function in the injured nervous tissue (post stroke, after severed nerves, etc.)
i. New stem cells (allogenic, endogenous from other own tissues or a combination of the two) implanted at the injury site and integrated in the existing neural networks
ii. Existing stem cells - endogenous neuronal precursor cells which are already in the brain-, stimulated to migrate, grow and differentiate in-situ to replace damaged neurons)
iii. Recovering the function of existing injury-stunned neurons by reversing oxidative and inflammatory- type damage and stimulating their metabolic function (mainly mitochondrial).
Even though the first strategy seems to have by itself exceptional individual results (the recovery from hemiparesis of Gordie Howe after allogenic neural stem cell infusion), the use of embryonic allogenic cells is a serious limitation in it becoming widespread because of ethical, cost and logistical considerations. The second strategy seems a more natural approach and may even yield better results long-term if we consider the risk of immune reactions; however in both cases the risk of neoplastic differentiation is yet to be determined. Another objection to treatments involving neuronal stem cell resides in the evaluation of its efficacy: in order to document the post-treatment progress it is difficult to directly evidentiate the results of neuronal regeneration in humans (strategies i. and ii.) which can be done best by sacrificing the respective tissues (surgical removal of respective brain tissue or post-mortem) and staining or immunohistochemical analysis of brain cells.
The third path, involving molecular stimulation of injured neurons and mitochondrial recovery, is easier to implement, has less risks (immunologic and oncologic) and may yield extra benefits for tissues other than the nervous ones (muscles, connective tissue, most cells in the body), considering that mitochondria are present in all cells and they play determinant roles in metabolism, aging, etc. It is important to mention here that improving mitochondrial function is a very important goal in the rapid recovery of athletes, regenerative medicine and anti-aging treatments and the above combination can be successfully used in such instances.
Finally, the importance of improving neuronal mitochondrial function (strategy iii.) was recently underscored by directly evidentiating the role of mitochondria in the axonal regeneration and function via the mitochondria-anchoring protein syntaphilin [15].
The improvements of neurological structure and function can be objectively followed up with surrogate markers from the cerebrospinal fluid (CSF) or blood - succinate dehydrogenase in peripheral blood lymphocytes, S100, visfatin, hemopexin, etc. - [16-19], which may correlate with the amplitude of the recovery processes in the nervous tissue, or by monitoring the electrical activity of the recovering brain areas [20, 21]. Clinicians have another very reliable and non-invasive tool to measure therapeutic progress, by documenting clinical improvements of neurological function with standardized, validated scales: National Institutes of Health Stroke Scale, Barthel Index, etc.; these can be employed independently or together with CSF or serum biomarkers.
The use of various substances to enhance recovery of damaged cells and neurons has been a longstanding goal in medicine and neurology. In their recent review [22] of stroke pharmacotherapy, Beristain and Golombieski underscored the need for better treatments for stroke survivors, more than half of whom have residual neurological impairment. The current standard pharmacotherapeutic arsenal is limited: antidepressants, acetylcholinesterase inhibitors, memantine, levodopa, methylphenidate, all of which work better in combination with physical and language therapy modalities. There are other therapeutic interventions available (cortical magnetic stimulation, stem cells, deproteinated veal serum, porcine brain lysate, complex polypeptides) and pharmacotherapies (citicholine, choline alfoscerate, ethylmethylhydroxypyridine succinate, 2-ethyl-6-methyl-3-hydroxypyridine L-aspartate, idebenone, meldonium), all of which are being or need to be studied in large clinical trials.
The deproteinated veal serum used in our combination (Actovegin) is being used by itself for treating stroke patients with good results since 1980’s (and more recently is being use for treatment of diabetic polyneuropathy, recovery of performance athletes and burn patients, arthritis), and there are many clinical trials documenting its efficacy [23-24].
The largest clinical study performed so far on Actovegin (503 patients, randomized, double-blind, multicentric) was completed in January 2016 by Takeda Pharmaceutical (ARTEMIDA Study) [25].
Actovegin was given intravenously for 20 days 2000 mg/day and afterward 1200 mg per day orally for 6 months; 503 patients were followed up for 1 year; of note there were slightly more females and more patients of younger age in the placebo group. There were significant improvements as measured by the Montreal Cognitive Assessment scale (starting 3 months after baseline) and Alzheimer Disease Assessment Scale – cognitive performance - (at 12 months). There were less patients with diagnosis of dementia at 6 and 12 months, improving scores (12.5 to 10.7) on Beck Depression Inventory; also on EuroQol EQ5D after 12 months; better percentage of patients in Actovegin group with severe mobility problems (3.2 vs 7.5%) and severe/unable self-care problem (2.9 vs 4.7%), and significant difference in moderate-severe-extreme anxiety (5.6 vs 11.8%). Serious adverse events were affecting 8.8% of Actovegin group and 6.7 of placebo treated patients; the most important difference was in incidence of ischemic stroke occurrence, which was 5.2% in Actovegin and 1.98% in placebo group.
DMSO has been in use for many decades, (it is FDA-approved for interstitial cystitis) mainly for protecting stem cells during cryopreservation in liquid nitrogen as it is one of the most powerful anti-oxidants and anti-inflammatory substances; its beneficial actions on stem cells was the main reason for its inclusion in our combination. Documented pathways [26] on which DMSO exerts anti-inflammatory actions are ERK1/2, p38, JNK and Akt; prostaglandin E2 and many other inflammatory cytokines are thus influenced by dimethylsulfoxide. In one recent study of such anti-inflammatory properties of DMSO [26], 13 of 15 studied chemokines were inhibited – interleukin -4 (IL-4), IL-1RA, IL-6, IL-8, IL-10, IL-12, IL-13, IL-17, along with TNF-α, VEGF, G-CSF, IFN-α and IFN-γ. The 2 cytokines unaffected by DMSO were interleukin 1-β and mФ inflammatory protein (MIP-1α).
It was recently showed that not all inflammation is deleterious in post-stroke recovery and that some inflammatory molecules and the cells modulated by them seem to have positive roles [27]; from this perspective the differentiated modulatory effects of DMSO can prove to have further importance.
As a final mention on DMSO and inflammation we may recall from the beginning of the article [3], that interferons play a role in neuronal function and plasticity, and the modulatory effects of DMSO on interferons (and probably on other molecules associated with inflammation) may have added positive effects on neurons.
The anti-oxidant properties of DMSO and glutathione are well known and seem to be very important for the survival and differentiation of stem cells [28] as they act directly through mitochondrial function to influence cell survival, differentiation and activities. The ability of DMSO, in conjunction with other antioxidant such as butylated hydroxianysole and most probably others, to stimulate transformation of mesenchymal cells in neuronal cells through inhibition of Wnt/β-catenin pathway is already known to take place [29].
Oxytocin is known to be a major modulator of complex behavior (social integration) and was shown to act on various cortical areas - cingulate cortex, medial prefrontal, etc. – and also to modify the activity of amygdala and other neural networks involving dopamine [30-32]. It has important effects on anxiety and novelty-seeking behavior, which are common problems post-stroke [33-35]. Moreover, recently was demonstrated a direct positive effect of oxytocin on various dopaminergic circuits (erection among others), and most importantly on neurogenesis and locomotion [36-39], rendering this substance very important in treatments aiming for neuro-psychologic improvement.
4. Conclusion
This intravenous combination adds an important tool to the therapeutic arsenal used in post-stroke recovery, yielding good clinical results within short time after administration and with little side effects.
While the gold standard therapy for ischemic stroke is clot removal with tPA (40% success defined as returning to independent living), to which recently was added stent thrombectomy (71% success rate) [40], it is essential that these interventions are performed within hours (3-6) after the stroke. This new treatment addresses a major temporal limitation of those treatments by showing good results even when administered weeks after the stroke. Perhaps more importantly it can be used in patients with hemorrhagic stroke or when the thrombolythic treatment cannot be used.
Another advantage is that it uses the patient’s own cells (recovered and/or regenerated) instead of infusing allogenic stem cells, so there are less costs and risks for the patients.
Finally, because it makes use of known and approved substances administered in a common modality (intravenous), this treatment is widely available and economical when compared to other therapeutic interventions.
This new treatment needs to be studied further, both in laboratory (pre-clinically) for detailing its molecular actions exerted on the neurons and glial cells, as well as through clinical studies which can show its efficacy and safety in comparison or in combination with physical therapy, other pharmaceutical substances, transcranial magnetic stimulation, endogenous or allogenic implants of the cellular (biological) or artificial (stembeads, electronic, etc.) kind, all of which can improve neurological function.
References