| 1. | ||
| 2. | ||
| 3. | ||
| 4. | ||
| 5. | ||
Degradative Effect of Aspergillus Species on Some Physiochemical and Chemical Parameters of Olein Fraction of Shea Butter
Esiegbuya O. D.1, 2, *, Okungbowa F. I.2, Obibuzor J. U.3
1Plant Pathology Division, Nigerian Institute for Oil Palm Research (NIFOR), Benin City, Nigeria
2Department of Plant Biology and Biotechnology, University of Benin, Benin City, Nigeria
3Biochemistry Division, Nigerian Institute for Oil Palm Research (NIFOR), Benin City, Nigeria
Email address
 (Esiegbuya O. D.)
(Esiegbuya O. D.) Citation
Esiegbuya O. D., Okungbowa F. I., Obibuzor J. U. Degradative Effect of Aspergillus Species on Some Physiochemical and Chemical Parameters of Olein Fraction of Shea Butter. American Journal of Food Science and Nutrition. Vol. 3, No. 4, 2016, pp. 45-51.
Abstract
The effect of some fungi species on the quality of the olein fraction of Shea butter was carried out with the aim of deducing their degradative effect on some of the physical and chemical quality of the olein fraction. This is important against the background of microbial contamination of processed Shea butter. The fungi were screened for extracellular lipase production using the cultural method in which Tween 80 was used as the sole source of carbon. The degradative effect of the identified fungi on the olein fraction of Shea butter was determined using the liquid fermentation method. While the ability of the fungal isolates to produce free fatty acids and peroxide from the olein fraction of the Shea butter was determined using the AOCS analytical method. The results of the effect of the different Aspergillus spp on the physical parameters of the olein fraction of Shea oil revealed that A. oryzae and A. niger were able to increase the yellow portion of the olein while A. persii, A. fumigatus and A. flavus were found to decrease the yellow fraction of the oil. The difference between values for experimental and control for pH and relative density were not significant while that of lipophilic activity of the isolates was highly significant. This ability of the fungal isolates to produce extracellular lipase on the fermentation medium resulted in the hydrolytic and oxidative rancidity of the olein fraction of Shea butter. These significantly alter the amount of the free fatty acids produced and peroxide release when compared with the control experiment. This study has revealed that the extracellular lipase produce by the fungal isolates used in this study have the potentials to cause partial hydrolysis of the olein fraction of Shea butter thereby leading to quality reduction.
Keywords
Extracellular Lipase, Shea Butter, Olein, Hydrolytic Rancidity and Oxidative Rancidity
1. Introduction
Lipases are special kind of esterases characterized by its unique ability to act upon emulsified substrate and hydrolyze glycerides to free fatty acids and glycerol (Gilbert, 1993). Ester synthesis is carried out in aqueous media in the presence of various lipases (Lacointe et al., 1996). Lipases occur widely in nature, but only microbial lipases are commercially significant (Mark et al., 2001; Hsu et al., 2002). It is well known that lipases are the most widely used enzymes in organic synthesis and more than 20% biotransformations are performed with lipases (Gitlesen et al., 1997). In addition to their role in synthetic organic chemistry, these also find extensive applications in chemical, pharmaceutical, food and leather industries (Gulati et al., 2005; Gunstone, 1999). Fungi characterized by being ubiquitous in distribution are highly successful in survival because of their great plasticity and physiological versatility (Iftikhar et al., 2010a; Iftikhar et al., 2010c). Fungi thrive well in habitats with environmental extremes because of their efficient enzyme systems. Among the varied mechanisms for fungi adaptability to environmental extremes and for the utilization of their trophic niche, their ability to produce extracellular enzyme is of great survival value (Gopinath et al., 2005).
Fats and oils are recognized as essential nutrients in both human and animal diet. Good health and life require dietary fats to provide a major source of energy, essential fatty acid, a vehicle for fat soluble vitamins and important components of cell membrane. Among all the microorganisms, fungi especially Rhizopus sp., Mucor sp., Aspergillus sp., Fusarium sp., and Penicillium sp., are preferable lipase sources. (Iftikhar and Hussain, 2002; Iftikhar, 2010). Aspergillus niger is among the well known lipase producer, mainly used in the in the industry (Pokorny et al., 1994; Undurraga et al., 2001.
The quality of fats and oils is dictated by several physical and chemical parameters that are dependent on the source of oil; geographic, climatic, and agronomic variables of growth in the case of plant oils as well as processing and storage conditions (Shahidi, 2005). In the case of Shea butter production, the fruits are collected from the ground but must not be allowed to stand for long on the ground as they germinate quickly. This is because the Shea fruit has no dormancy period, it germinates within few days of dropping from the tree (Jøker, 2000).
The state of a plant material prior to processing could affect or modify all or some of the food qualities parameters besides other factors such as processing methods and conditions (Olaniyan and Oje, 2007). It has been reported that changes in the expression of various fatty acid metabolizing enzymes can result in changes in seed oil compositions (Napier, 2007; Knutzon, 1992). For example, the predominant off flavour perceived in some products made from soybeans comes from the activity of endogenous lipoxygenase enzymes found in soybeans (Iassonova et al., 2009).
According to Obubizor et al. (2013) depletion of butter in the germinated kernel, was due to the butter being mobilized and consumed during the germination process. This led to depletion in the value of the lipid content of Shea kernel, and elevated the FFA by 7-folds, the peroxide value (PV) by 81, while iodine value decreased when compared to the ungerminated. Low FFA improves shelf life of butter. Such butter therefore attracts a premium price. The elevated free fatty value could be attributed to the germination process even though it is a known fact that hydrolysis could proceed via microbial, enzymatic and autocatalytic pathways. According to the author, for maximum butter yield, all the conditions necessary for Shea kernel germination must be eliminated or reduced to the barest minimum and prompt processing of the collected fruit must be considered as an important requirement.
According to USAID (2004), Nigeria is the major producer of Shea nuts in Africa but the export rate of the Shea nuts and Shea butter is far below its potential. This was attributed to low quality of the processed Shea nuts and Shea butter. Adomako, (1985), previously reported that traditionally processed Shea butter have high iodine number and high free fatty acid value. This occurrence has been reported to be indications of partial hydrolysis of the fat caused by fungal spoilage during processing and storage (Aye, 1989).
In Nigeria, Shea butter is normally used in topical and oral applications especially in children. In the oral application, a small amount of the butter is melted over the fire in a spoon and a pinch of salt added and given as expectorant. This is done without any knowledge of its microbial load.
In order to contribute towards improving on the quality of Shea butter, there is also need to generate knowledge on the influence of particular group of microbes on the quality of the processed Shea butter. This is important against the poor quality and international market penetration of Shea butter processed in Nigeria.
The generated information can be used by the communities, development partners, researchers, individual processors and government agencies to create policy for Shea butter production in Nigeria, improve income generation and food security among rural communities particularly in the Shea butter belt of Nigeria in addition to improving marketing and utilization of Shea butter.
This study is therefore aimed to determine the effect of the partial hydrolysis of some fungi on some quality parameters of the olein fraction of Shea butter.
2. Material and Methods
a. Source of microorganisms
A pure culture of A. niger, A. niger (aggregate), A. fumigatus, A. persii, A. flavus and A. oryzae obtained from the Plant Pathology Division of the Nigerian Institute for Oil Palm Research (NIFOR) where it was originally isolated from processed Shea butter and kernels collected from Kwara and Niger States. The identification of the isolates were also previously confirmed by CABI Identification service UK. It was then sub-cultured on Potato Dextrose Agar (PDA) supplemented with 1ml of 40g/ml gentamycin.
b. Screening for lipase producing fungi
The lipase activities of the identified fungi were screened according to the methods described by Akano and Atanda, (1990). Tween 80-agar plates were used for the isolation of lipolytic fungi. Tween 80 was separately sterilized and added into the rest of the autoclaved medium, and the pH was adjusted to 6.0. About 10 mL medium was poured into each Petri dish and inoculated. Lipolytic activity was indicated by the appearance of a visible precipitate, resulting from the deposition of crystals of the calcium salt formed by the fatty acid liberated by the enzyme, or as a clearing of such a precipitate around a colony due to complete degradation of the salt of the fatty acid. At regular intervals of 24 h incubation, each plate was examined and measurements of zones were taken to monitor lipolytic activity (Gopinath et al., 2005). The strains showing the highest activity were selected.
c. Preparation of fungal mycelia disc
The centre of each PDA plate containing each of the fungal isolates was punched with a cork borer of a 0.5 mm, 1.0mm, 1.5mm and 2.0mm in diameter. The fungal disc form was then inoculated into the fermenting mentioned below.
d. Screening for free fatty acid and peroxide production
The isolated fungal strains were screened for their ability to release free fatty acids and peroxide from the olein fraction of Shea butter using the liquid fermentation method. The screening was carried out in 250 ml Erlenmeyer flasks containing 100 ml of fermentation medium g/L (Olein fraction of Shea butter 5mls; Glucose, 10.0g; K2HPO4, 2.0g; NaNO3, 0.5g; MgSO4.7H2O, 0.5g; pH 7.0). Lipolytic efficiency was monitored by the rate of free fatty acids and peroxide production.
e. Determination of rate of production of free fatty acids and peroxide
The release of free fatty acid and peroxide from the olein fraction of the Shea oil by fungi strains was determined by analyzing the supernantant of the medium. The mean of the amount of the free fatty acid produced were recorded for each of the three replicates by subtracting 0.5mm, 1.0mm, 1.5mm and 2.0mm of the initial amount of free fatty acid from the total amount of the produced free fatty acid. The amount of peroxide produced was then determined using the least size of the fungal disc that was able to cause free fatty acids release.
The free fatty and peroxide from each of the fungal mycelia disc was thus determined as illustrated below.
Calculations
Free fatty acid ₌![]() (1)
(1)
where
V ₌ Standard volume of sodium hydroxide used.
M ₌ Exact concentration in mole per litre of sodium hydroxide.
m ₌ mass of test substances
Acid value ₌ 2×FFA (2)
Peroxide value ![]() (3)
(3)
Where
V ₌ volume of sodium thiosulphate used.
Vo ₌ volume of sodium thiosuphate used for the control.
c ₌ concentration of the sodium thiosuphate.
m ₌ mass of the test substances.
f. Determination of physical parameters
Optical density, density, lovibond colour and pH were also determined using the standard method of analysis of AOCS (1997).
Determination of density
The density of the olein fraction of the Shea oil was determined with the aid of a density bottle, by weighing the density bottle without any content in a weighing balance and weight recorded. This represents the initial weight (w1). After which, the density bottle was filled with the sample and the final weight also determined in a weighing balance representing the final weight (w2).
Density of sample₌ w2- w1
Determination of Colour: The olein fraction of the Shea oil was placed in a cuvet and analyzed using a Lovi bond –Tintometer model E, S. No. 5064E, England. The colours of red, yellow and blue units were adjusted until a perfect colour match was obtained. The unit value of the colour with the lowest unit was subtracted from the colours leaving two units which were then used to describe the colour of the sample (AOAC, 1997).
Determination of pH
After incubation of the fungal mycelia disc in the fermenting medium, the pH of the supernatant of the centrifuged sample which is the remaining of the olein fraction of the Shea butter was then determined with the aid of a pH meter.
Determination of optical density
The optical density of the remaining portion of the olein fraction of the Shea butter was determined by first calibrating the spectrophotometer with a blank sample, after then the control sample olein fraction of the Shea oil was then used to determined the optical density of the sample by scanning the peak wavelength at which light will pass through the sample. The obtained peak wavelength was then used to determine the wavelength of the experimental samples.
Determination of the lipophillic ability of the isolates
Conical flask containing mycelia growth of the test organisms and the fermentation medium in the conical flasks was then transferred into graduated test tubes. The test tubes were centrifuged for 20mins at 2000rpm. The two different separating layers were read.
Calculations:
Water soluble portion ₌ Vi-Vo
Where Vi ₌ reading mark of upper layer.
Vo ₌ reading mark of lower layer.
g. Statistical analysis
Data obtained were subjected to analysis of variance (ANOVA), and means were separated using the Duncan’s multiple range (DMR) test at 5% level of significance (Ogbeibu, 2005).
3. Results
h. Effect of Aspergillus species on the quality of the olein fraction of shea oil
The results of the effect of the different Aspergillus spp on some physical parameters of the olein fraction shown on table 1 below revealed that there was a significant difference on the lipophillic activity of the isolates and no significant difference was observed for the pH, optical density and density values. This result indicates that the identified Aspergillus spp in this study did not have any effect on the pH and optical density and density of the olein oil. However, A. niger agg), A. fumigatus, A. persii and A. flavus were found to decrease the yellow portion of the olein of the Shea butter while the other A. niger isolated from Kwara State and A. oryzae increases the yellow portion.
The A. flavus detected in this study were also found to utilize the olein fraction of Shea butter thereby significantly reducing its volume in the test tubes as shown on plate 1 below. This clearly indicates its lipohillic ability.
Table 1. Effect of Aspergillus spp on some physical parameters of the olein fraction of Shea butter.
| Parameters | Control | A. niger(agg) | A. flavus | A. flavus | A. fumigatus | A. oryzae | A. niger | A. persii |
| Lipohillic (ml) | 9.7 ±1.2 | 9.5 ±1.2 | 2.7 ±1.2 | 2.9 ±1.2 | 9.7 ±1.2 | 9.6 ±1.2 | 9.6 ±1.2 | 9.5 ±1.2 |
| pH | 6.56 ±0.2 | 5.59 ±0.2 | 5.70 ±0.2 | 5.83 ±0.2 | 5.51 ±0.2 | 5.27 ±0.2 | 5.82 ± 0.2 | 5.12 ±0.2 |
| Density | 0.93 ±0.01 | 0.965 ±0.01 | 0.965 ±0.01 | 0.96 ±0.01 | 0.965 ±0.01 | 0.954 ±0.01 | 0.97 7 ± 0.01 | 0.977 ±0.01 |
| Colour | 3R, 16.2Y | 3R, 9Y | 4R, 7Y | 4R, 8Y | 3R, 7Y | 4R, 40Y | 3R, 20Y | 3R, 10Y |
| Optical density | 2.99 | 2.99 | 2.54 | 2.60 | 2.88 | 2.53 | 2.31 | 2.38 |
i. Effect of Aspergillus spp on some chemical parameters of the olein fraction of Shea butter
The results of the effect of the different inoculums sizes of the different Aspergillus spp on the chemical parameters (free fatty acids and peroxides) of the olein fraction of Shea butter revealed A. oryzae and A. flavus to be the highest producers of free fatty acids of 1.2%/5g of the olein fraction of the Shea butter. While A. niger (agg) was the least with 0.8%/5g of the oil. There was significant between the percentage amounts of free fatty acids produced by the different inoculums sizes when compared with the control experiment. This indicates that the amount of degradation of the chemical parameters is dependent on the spore size.
The amount of acid detected in the olein fraction as shown on figure 2 below is an indication of the hydrolytic activity of the microorganisms.
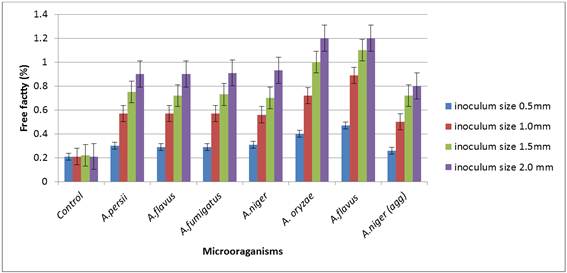
Figure 1. Lipase activities of different inoculums sizes of Aspergillus species on the oleic fraction of Shea butter.
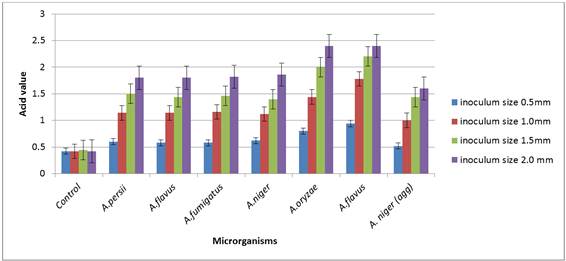
Figure 2. Acid production of different inoculum sizes of Aspergillus species on the olein fraction of Shea butter
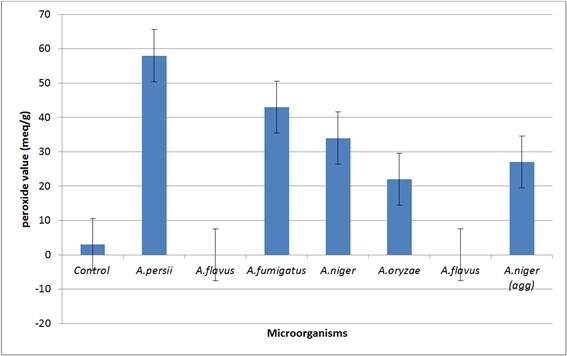
Figure 3. Peroxide value of different microorganisms isolated from Shea buttThe result of the degradative effect on the chemical parameters of the olein fraction of Shea butter shown on Table 2 below indicates that different Aspergillus spp base on the ability for them to produce the enzyme lipase has varied effect of hydrolytic, oxidative and lipohillic on the olein fraction of the Shea butter.
Table 2. Type of spoilage caused by the different Aspergillus spp.
| Isolate | State | Type of spoilage |
| A. niger (agg) | Niger | Hydrolytic /oxidative rancidity |
| A. flavus | Niger | Hydrolytic rancidity /Lipophillic |
| A. niger | Kwara | Hydrolytic /oxidative rancidity |
| A. persii | Kwara | Hydrolytic /oxidative rancidity |
| A. fumigatus | Niger | Hydrolytic /oxidative rancidity |
| A. oryzae | Niger | Hydrolytic /oxidative rancidity |
| A. flavus | Kwara | Hydrolytic /oxidative rancidity |
The results of the effect of the different Aspergillus spp on peroxides formation shown on figure 3 below revealed A. persii to be the highest producer of peroxide. The high amount of peroxides produced by the microbe is an indication of its oxidative rancidity. Aspergillus flavus which recorded high amount of free fatty acids production did not record any amount of peroxide.
4. Discussion
According to the ASBI (2002), Shea butter can be degraded by heat, moisture and microbes. The amount of free fatty acids present in Shea butter tells us the degree of destruction to the moisturizing fraction of the butter. The authors also stated that the quality of the moisturizing fraction can be determined by review of the content of free fatty acid and peroxide. When free fatty acids and peroxide are greater than 2% and 2 meq per kg respectively, significant damage has occurred. The free fatty acids tell us the integrity of the moisturizing fraction; therefore, low free fatty acid is consistent with high quality Shea butter. While peroxide test tells us the amounts of peroxides have been produced by oxygen.
Shea butter with free fatty acids and peroxide value stated above, trigger concern and caution in buying and selling.
In this study, the olein fraction of the Shea oil was use because it is the liquid part portion that is being used by cosmetics and pharmaceutical industries. The activities of the isolated Aspergillus species on the olein fraction of the Shea oil were found to increase the free fatty acids and peroxides above the set limit. This indicates that microbes contribute to partial hydrolysis of the Shea butter thereby reducing its quality. The various Aspergillus spp detected were found to cause both hydrolytic and oxidative rancidity of the olein fraction of Shea butter thereby leading to its deterioration. The ability of the isolates to degrade to olein fraction of the Shea butter was due to their ability to produce lipase which was determined in this study using the culture base techniques.
Negedu et al. (2011) stated that fungi can cause increase in the free fatty acids (FFA) and peroxides of the oil in a liquid medium to varying levels. This is due to their specificity and preferences in the utilization of component of the fatty acids as carbon sources Chakrabarti, (1987).
The ability of the different fungal strains to utilize the olein fraction of the Shea butter to produce vary degrees of FFA and peroxide further confirms their specificity and preferences of components of fatty acids as carbon sources. In this study A. flavus which recorded one of the highest amounts of free fatty acid production recorded no amount of peroxide production. This implies that the there is variation in the ability of a fungal isolates to produce FFA and peroxide.
According to Kaul et al., (2008), presence of peroxide has been reported to indicate increase in iodine value due to oxidative hydrolysis. In this study, the iodine value was not determined because it is not a major parameter for quality determination of Shea butter. Anhwange et al., (2004), also reported that high peroxide value could be responsible for the development of rancidity in the oil (Since rancidity can affect the nutritional quality, physical appearance, flavor and safety of oil and is of great concern to consumers (Rathjen et al., 1997), efforts should be made to minimize the development of rancidity in Shea oil.
In addition to peroxides, increasing iodine value, it also increases the viscosity of Shea oil extracted by traditional boiling and cold pressing methods. Since viscosity is a physical parameter that determines quality of vegetable oils, increase in viscosity is normally caused by oxidation and polymerisation of vegetable oil. The viscosity of the olein fraction of the Shea butter was not determined in this study.
Several authors have reported on the ability of fungi to cause hydrolysis of fat and oil to release free fatty acids and peroxide but there has be no report on literatures on the impact of these chemical reactions caused by fungi on the physical quality of fats and oil. According to Lewis (1990), physical parameters play an important role in determining the quality of any oil because they give physical specification for description of the oil. These are quick parameter for characterization of oils and assessment of the level of purity.
Shea butter has been reported to have a pale yellow, cream or grey colour solid at room temperature and refractive index of 1.467 (Adikini, 2002). However, a report by Stryer (1988) indicates that variation in the physical parameters of vegetable oils might be associated with changes in the un-saturated fatty acids due to oxidation, polymerization and isomerisation. Colour on the other hand can be a good indicator of vegetable oil quality. The change in the colour of vegetable oil is mainly attributed to peroxidation, pigmentation or contamination (Lewis, 1990). The effect of the different microbes on the colour of the olein fraction of the Shea oil was observed to cause the differing in the red–yellow fractions. This could probably be due to pigmentation released by the microbes into the fermenting medium.
The ability of the A. flavus detected in this study, to utilize the olein fraction of the oil is a clear indication that the fungi can degrade the olein fraction of the Shea butter into substances soluble in water in addition to its quality reduction potential.
5. Conclusion
This study has shown that the extracellular lipase produce by fungi can also results in the poor physical and chemical quality of processed Shea butter. For the quality of the Nigerian Shea butter to be improved on, poor postharvest and processing practices which encourages microbial contamination of the different processing stages should be avoided. Research is still needed on production, characterization and purification of their enzyme through optimization of process parameters such as pH, temperature and various substrate utilizations.
References