| 1. | ||
| 2. | ||
| 3. | ||
| 4. | ||
| 5. | ||
| 6. | ||
Lycosome Technology: Advances and Perspectives
Ivan M. Petyaev*
Lycotec Ltd, Granta Park Campus, Cambridge, United Kingdom
Email address

Citation
Ivan M. Petyaev. Lycosome Technology: Advances and Perspectives. American Journal of Food Science and Nutrition. Vol. 3, No. 1, 2016, pp. 18-23.
Abstract
Lycosome technology represents a new development in the modern biotechnology directed at improvement of bioavailability of nutraceuticals and pharmaceuticals as well as their organ-specific delivery. Lycosome technology exemplifies a nano-microencapsulation methodology using unique physicochemical properties of lycopene as an organic compound. Lycopene is a symmetrical non-polar tetraterpene compound containing 8 isoprene units with 13 double bonds essential for its antioxidant activity. Lycopene gains polarity and ability to interact with phospholipids upon cis-isomerization caused by heat, light exposure and pH changes. It promotes the development of thermodynamically stable and highly rigid lycopene-phospholipid biofilms suitable for coating and microencapsulation of different amphiphilic compounds and finally formation of lycosomes – nano-microdimensional particles with partial resistance to gut environment. The lycosome particles are composed of external layer and internal domain. The internal domain contains a bioactive compound of interest which needs to be delivered and distributed in the human body. It is protected by external layer containing lycopene, a hydrophobic carotenoid used as a core-forming substance. Moreover, external layer contains an amphiphilic phospholipid (phosphatydilcholine) serving as a chaperone for lycopene, and emulsifying as well as hydrophilyzing agent. Different regimens of microemulsification modus operandi which involves spray drying, ultrasound, supercritical CO2 fusion, and other physical factors allow to obtain various lycosome formulations of nutraceuticals and pharmaceuticals as well as functional food products useful in prevention and management of cardiovascular disease and type 2 diabetes mellitus. Moreover, lycosome technology can be used for production of fortified food products for example functional dark chocolate, margarine and butter.
Keywords
Lycosomes, Lycopene, Technology, Functional Food
1. Introduction
Current decade reshapes human nutrition as we know from the standpoint of bioavailability of nutrients. As consumers become more and more conscious about the nutritional characteristics of food, there is an exponential demand for quality food products conferring best nutritional value and some health benefits. However, Mother Nature remains very protective to all living beings and shelters human organs and tissues from xenobiotic overaccumulation by limiting absorption of nutrients and pharmaceuticals in the gut and/or effectively disposing them via excretory pathways. These principle is a keystone component in understanding of evolutionary and overall biological significance of intestinal bioavailability as a phenomenon of organic life. However such safeguarding the human cells from potentially toxic nutrients has a certain trade-off value. Some non-toxic phytochemicals retain low bioavailability rate despite their essential role in human health and enormous potency comparable pharmaceuticals. These compounds are often referred as nutraceuticals.
Poor aqueous solubility of xenobiotic bioactive molecules is a most common reason of poor intestinal permeability and overall bioavailability of exogenous organic compounds [1]. Moreover many xenobiotics derived from food (nutraceuticals) or artificially synthetized compounds (pharmaceuticals) are existent in acidic or basic form which compromises their intestinal bioavailability rate. Therefore salt formation is a common approach to increase bioavailability rate of many xenogeneic bioactive compounds [2].
Food matrix structure deeply affects bioavailability of nutraceuticals. For example, lycopene, an important carotenoid with extremely potent antioxidant properties, is known to be compartmentalized in a crystalline form within poorly digestible ultracellular organelles of plant cells called chloroplasts [3]. It makes lycopene virtually unavailable from a raw matrix of tomatoes, watermelons, carrots and papaya [4].
Particle size reduction to nano- or microdemensions is proven to be one of effective ways to enhance bioavailability of insoluble nutraceuticals and pharmaceuticals. Nano- and micro-dispersions have an enhanced permeability rate through intestinal epithelium even in case of completely insoluble biologically active compounds [5]. It is especially tempting to consider an engineering attempt to construct nano-microparticles with functional coating when nano- and/or micro-dispersions of bioactive compounds are covered with a ligands promoting an active trans-epithelial transport in the intestine.
Recently developed Lycosome TM technology (Lycotec Ltd, Cambridge, UK) may serve as a good example of successful implementation of this approach.
2. Lycosome Technology
Lycosome technology is based on some unique physicochemical properties of lycopene as an organic compound. Lycopene is a 40-carbon acyclic water insoluble tetraterpene compound containing 8 isoprene units with 13 linearly aligned double bounds [3]. Lycopene is present in nature in an all-trans isoform, named often as all-E-lycopene. Thermal processing, some physical factors, acidic pH as well as intestinal digestion of raw lycopene-containing products promotes cis-isomerization of lycopene [6]. In human body lycopene is represented predominantly by various cis-isomers (designated as (Z)-lycopenes). It is suggestive of that cis-transformation is crucial for intestinal absorption since it confers polarity to lycopene molecule [7]. Exposure to heat, light and oxygen facilitates cis - isomerization nut may cause irreversible degradation of lycopene to a number of end-products [8]. Distinctive red color to lycopene crystals is attributable to chromogenic C=O bonds in the lycopene structure and are essential for antioxidant properties of lycopene which is a major functional feature of the compound [3,7]. Due to presence of double bonds lycopene has highest oxygen radical quenching ability exciding antioxidant properties of carotene at least two times [3].
Lycopene can interact efficiently with phospholipids and can be introduced into structure of phosphatidylcholine membranes conferencing them decreased fluidity, better stability and increased the order of the alkyl chains. [9]. Lycopene-phospholipid interactions are essential for lipophilic properties of lycopene and its ability to be integrated and transported by lipoproteins [3,10]. Thorough investigations of lycopene-phospholipid interaction allowed us to establish conditions for engineering thermodynamically stable lycopene-phospholipid membranes and complexes in heterogeneous phase. These complexes are comprised of lycopene and phosphatidylcholine derived from dietary sources (tomatoes and eggs) and suitable for coating, encapsulating or embedment of different amphiphilic substances on nano-microdimensional scale. The lycosome technology was recently reviewed among others innovative approaches in the "World Frontiers in Biomedicine" [11].
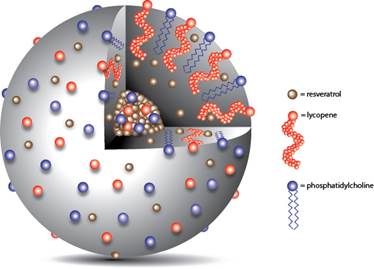
Figure 1. Lycosome Structure.
It employs microemulsification modus operandi with spray drying, supercritical CO2 ultrasound and other physical methods (12). The lycosome particles are composed of external layer and internal domain (Figure 1). The internal domain contains a bioactive compound of interest (for example resveratrol, a dietary polyphenol) which needs to be delivered and distributed in the human body. It is protected by external layer containing lycopene, a hydrophobic carotenoid used as a core-forming substance. Moreover, external layer contains an amphiphilic phospholipid (phosphatydilcholine) serving as a chaperone for lycopene, and emulsifying as well as hydrophilyzing agent. It increases by this means the intestinal absorption of lycosomes. The lycosome particles could be present in a powder form or in an oil suspension. These particles are relatively more resistant to gastrointestinal digestion and provide cargo molecules some level of protection from acidic degradation in stomach. As a result, more cargo molecules can reach the sites of their absorption in unmodified form. Moreover, some lycosome particles can penetrate gastro-intestinal barrier in unmodified form and deliver additional amount of cargo bioactives into the circulation.
In addition, lycosome technology can be used for microencapsulation of bioactive molecules in situ within different food matrixes / products. Adjustments in microemulsification modus operandi during tempering process may promote formation of lycopene-phospholipid layers around hydrophobic chocolate crystals. This technology was successfully applied to create coco-lycosomes of functional dark chocolate with enhances cardio-vascular and lipid management beneficial properties [13].
Figure 2. Lycosome-formulated Dark Chocolate with Blueberry Anthocyanins and Chocolate Crystals.
It has also been demonstrated that this technology can facilitate introduction and co-assembly of other bioactive molecules into chocolate crystals to enhance their intestinal absorption. Such co-crystallization results not only in the more efficient fortification of chocolate matrix with new molecules, but due to the lycopene protecting coating potentiate bioavailability and efficacy of the incorporated ingredients of this product. An example of successful application of the lycosome technology to incorporate blueberry anthocyanins into chocolate crystals and provide protective coating with lycopene is presented in the Figure 2.
Versatility of lycopene-phospholipid complexes in creation of either reverse micelles of lycosomes, or its use in creating protective coating of nutrient crystals, opens countless possibilities for engineering lycosome-formulated products.
3. Lycosomes and Heart Disease
Cardiovascular disease (CVD) represents a leading cause of global mortality and morbidity [14]. Although changes in diet and life style may have significant impact on initiation, development and outcomes of heart disease, a pharmacological approach remain a main strategy to confront the global CVD epidemic. Abnormalities of lipid homeostasis and increased cholesterol levels in the blood form a keystone mechanisms of pathogenesis in cardiovascular disease. Inhibitors of cholesterol biosynthesis, called otherwise statins
or HMG-CoA reductase inhibitors, represent a most widely class of drugs used for prevention and treatment of heart disease [15]. Despite unquestionable clinical benefits translating into significant reduction of stroke and heart attacks, about 25% of patients treated with statins experience various side effects [16]. It requires dose reduction which in turn minimizes therapeutic benefits of the statin treatment and negatively affects patient compliance to the treatment schedule. It has to be mentioned, that statins belong to a class of organic compounds with poor aqueous solubility and intestinal bioavailability rate. As we recently shown, lycosome technology can be efficiently implemented in statin biopharmacology and may increase bioavailability of statins. A newly proposed (Lycotec Ltd, UK) lycosome formulation of simvastatin, designated as Lycosimvastatin, has been shown in our work [15] to have a superior ability in the reduction of LDL-cholesterol and ApoB in hypercholesterolemic patients as compared to unmodified Simvastatin. Detailed analysis of Lycosimvastatin biopharmacology reveals, that lycosome formulation of Simvastatin promotes intestinal absorption and intrahepatic delivery of the drug to the liver possibly via receptor-mediated mechanism via very low density lipoproteins (VLDL) and carotenoid receptors expressed in hepatocytes. Ingestion of lycosome-formulated drug is accompanied by incorporation of lycosome particles into VLDL synthetized into enterocytes and preferential delivery of Simvastatin into liver cells, a main target cell population for action of HMG-CoA reductase inhibitors. Therefore, implementation of lycosome technology allows to redirect the drug trafficking from extrahepatic tissues, where statins have a toxic effects, to the liver minimizing thereby statin toxicity. Hence, it provides an important solution for prevention of statin toxicity and helps to maximize therapeutic benefits of statin treatment.
Another important application of lycosome technology in prevention and treatment of heart disease came out in our work with whey protein, a byproduct of cheese manufacturing. Whey protein is a complex substance containing various peptides and proteins, known to affect cardiovascular functions [17]. As we showed in a clinical trial [18], that administration of the lycosome formulation of a whey protein isolate to patients with prehypertension resulted in reduction of their systemic blood pressure, improvement of the plasma lipid profile, and the inflammatory status. Neither the control group, which administered unmodified whey protein isolate, nor the placebo group did not have such effects. The reduction of plasma triglycerides and cholesterol fractions and almost two-fold decline in C-reactive protein (CRP) and inflammatory oxidative damage (IOD) levels, as well as an increase in nitric oxide (NO), tissue oxygenation [StO(2)], and flow-mediated dilation values constitute the most significant benefit/outcome of the treatment with the lycosome formulation of the whey protein isolate.
4. Lycosomes and Diabetes
Growing epidemic of type 2 diabetes mellitus (T2DM), affecting 382 million people worldwide represents another challenge for modern medicine and health care [19]. T2DM is a lifestyle disease caused by excessive caloric intake and physical inactivity [20]. Therefore nutritional intervention as well as physical exercise becomes most favorable methods for T2DM management. Recent attempts to apply Lycosome technology to T2DM management lead to proposal of new nutraceutical formulation of resveratrol for diabetic patients. Resveratrol is a polyphenol from grapes and red wine highly susceptible for oxidation and metabolic transformations by gut microbiota. We have assumed [21] that resveratrol treatment can be very efficient in the treatment of complications of T2DM patients. This prediction was based on multiple lines of evidence suggesting that resveratrol plays a crucial role in restoration of insulin sensitivity, microcirculation, tissue regeneration, function of peripheral nerves and production of cytokines [21]. A lycosome formulations of trans-resveratrol with high absorption rate and stability to oxidation was proposed and successfully tested in T2DM patients with diabetic foot syndrome [22]. According to our results [22], treatment with lycosome formulation of resveratrol promoted healing of the diabetic foot ulcers as compared to the placebo group and improved performance of the patients in the foot pressure test. A statistically significant decline in the plasma fibrinogen level, but not C-reactive protein, was also found in the resveratrol-treated patients. Moreover, all patients enrolled in the study had better parameters of glycemic control and higher resveratrol levels in blood. Therefore, the application of products based on lycosome technology into treatment of patients with T2DM could provide a new effective option for management of diabetic complications and development of T2DM. Creation of new lycosome-based nutraceuticals and pharmaceuticals for prevention and treatment of T2DM are currently under development by Lycotec Ltd. (Cambridge, UK).
5. Lycosomes and Functional Food
There is an emerging group of newly developed food products with alleged health benefits beyond their nutritional value. However their medicinal efficacy needs is yet to be confirmed in reasonably large clinical settings. Dark chocolate produced from the beans Theobroma cocoa, is a millennia-old product with suspected health benefits. There is a solid scientific evidence, that cocoa polyphenols and methylxanthines – two major classes of organic compounds with confirmed physiological action, induce favorable changes in nitric oxide production, insulin sensitivity and cardiovascular function in vitro systems [23]. However, clinical trials on dark chocolate use generate not always reproducible results on cardiovascular benefits of dark chocolate, presumably and in part due to poor intestinal absorption of cocoa bioactives [23]. To overcome this problem, the lycosome technology was applied to protect cocoa polyphenols in the dark chocolate matrix. It was shown that lycosome-formulated dark chocolate promotes absorption of cocoa polyphenols and provides higher levels of epecatechins in blood of the volunteers. As we reported [13], lycosome-formulated dark chocolate was more efficient in reducing diastolic blood pressure when compared with the regular dark chocolate. Its effect on the systolic blood pressure was less prominent. However, the L-tug formulation was the only formulation of DC which affected serum lipids. There was a reduction in total cholesterol with corresponding decline of low-density lipoprotein (LDL) cholesterol after 30-day treatment period. Similar decline was seen in serum triglycerides.
Therefore application of the lycosome technology allows to exuberate well known functions of dark chocolate, such as the effect on systemic blood pressure as well as to uncover previously unknown medical benefits of dark chocolate use, in particular, its effect on plasma lipid profile.
6. Conclusion
As shown above, lycosome technology, a new microencapsulation technology applicable for nutraceuticals and pharmaceuticals, has been proven to have multiple useful applications for food and medical industries. Recent advances in nutrition make it difficult to navigate among hundreds of patents and publications addressing the problem of nutritional value of food. However Lycosome delivery technology differ from any other technologies improving bioavailability of food ingredients. First of all, unlike many other microencapsulated products lycosome particles and lycosome biofilms contain only food-derived natural ingredients resolving thereby a biosafety issue. Lycopene, used as a core forming agent in lycosome particles, is derived from tomatoes, whereas phospholipids (phosphatydilcholine) originates from egg yolk. Moreover, beside exceptional biosafety record and obvious impact on bioavailability of other nutraceuticals, lycopene inherently confers some measurable degree of cardiovascular and neoplastic protection to lycopene-containing food formulations. Cholesterol-lowering, and antitumorigenic actions of lycopene arising from its antioxidant properties are reported in multiple independent clinical trials.
Secondly, microencapsulation protocols based on lycosome technology open new horizons for targeted organ-specific delivery of nutraceutical and possibly pharmacological agents. Lycopene is known to be a powerful ligand for carotenoid receptors abundantly expressed in liver, brain and some other vital organs. Therefore upon ingestion lycosome particles become bound and internalized in the tissues where carotenoid receptor is expressed at highest degree creating thereby a targeted traffic of nutraceuticals in the human body via network of carotenoid receptors. Selective hepatic delivery of nutraceuticals and certain drugs is essential, since the liver is a central organ responsible for biotransformation and action of bioactive substances of foreign origin. Some nutraceuticals and drugs work exclusively through hepatic mechanisms and do not display any effect unless they are available in liver.
Nevertheless, there are greater and even more exciting perspectives for lycosome technology. Western diet, rich in saturated fat and deficient in vegetable and sea food products, becomes recently a predominant dietary pattern around the World. Since high saturated fat consumption is irrevocable feature of our present and seemingly future, there are multiple ongoing efforts to develop food technologies minimizing harmful effects of saturated fat. Versatility of lycopene-phospholipid films and countless possibility of modifications of lycosome formation protocol, opens the door for lycosome technology use in formulation of saturated dietary fat products, such as margarine, butter and vegetable oils. Selective trafficking of dietary lipids and cholesterol derived from saturated fat to the liver, responsible for elimination of fat excess in human body, may attenuate lipid accumulation of in blood vessels reducing in this manner the risk of atherosclerosis. Therefore lycosome-formulated butter, margarine and oils may bring eventually the lycosome technology on the dinner plate of the consumers, promoting their cardiovascular health and well-being.
References