Proximate Analysis and Phytochemical Screening of Fluted Pumpkin (Telfairia occidentalis) Pod
Akwukwaegbu P. I., Peters D. E.*, Wegwu M. O.
Department of Biochemistry, Faculty of Science, University of Port Harcourt, Choba, Rivers State, Nigeria
Email address

(Peters D. E.)
*Corresponding Author
Citation
Akwukwaegbu P. I., Peters D. E., Wegwu M. O. Proximate Analysis and Phytochemical Screening of Fluted Pumpkin (Telfairia occidentalis) Pod. American Journal of Food, Nutrition and Health. Vol. 1, No. 1, 2016, pp. 1-6.
Abstract
Proximate analysis and phytochemical screening of fluted pumpkin (Telfairia occidentalis) pod was investigated using standard analytical methods. Results of the proximate analysis showed moisture (43.18±0.59%), Ash (6.26±0.59%), crude fibre (15.43±0.01%), lipid (7.43±0.01%), crude protein (10.73±0.20%) and carbohydrate (16.97±0.21%); while phytochemical screening gave tannins (6.41±0.95%), saponins (0.83±0.01%), alkaloids (0.66±0.01%), flavonoids (1.48±0.01%), cardiac glycosides (9.37±0.02%), oxalate (3460.00±0.01mg/100g), phytate (0.65±0.02%) and phenol (0.63±0.00%). It can be concluded that pumpkin pod waste has high moisture, fibre and tannins with low phytate, phenol and oxalate contents.
Keywords
Pumpkin Pod, Waste, Proximate, Phytochemicals
1. Introduction
Fluted pumpkin (Telfairia occidentalis); a member of Cucurbitaceous family is one of the commonly consumed leafy and seed vegetables in Nigeria. Cucurbitaceae is a plant family commonly known as gourds or cucurbits and includes crops like cucumbers, squashes (including pumpkins), luffas melons and water melons. The family is predominately distributed around the tropics, where those with edible fruits were amongst the earliest cultivated plants in the world. The fruit is often a kind of berry called a pepo [1]. The cucurbita species are from primarily Africa and South America [2]. The fluted pumpkin is no longer known in the wild, but most likely originated in West Africa’s high rainfall forest belt. The largest diversity in plant populations can currently be found in Imo state and surroundings areas in South-eastern Nigeria.
For the Igbo people it is the most popular leafy vegetable. The term fluted refers to the shape of the female flowers which resemble a flute. It is also called fluted gourd and in Nigeria, the Igbo people call it Ugu, the Yoruba people use Ugwu, and in Cameroon it is referred to as Ekobon. The main use of T. occidentalis is as a leaf and seed vegetable. The leaves are rich in iron and are used to cure anaemia. The oily seeds also have lactation promoting properties and are widely consumed by nursing mothers. The roots are known locally as potent human, fish and rodents poison. The plant also contains considerable amount of anti-nutrients such as phytic acid, tannin and saponin which could also have some health benefits [3, 4]. Previous studies usually focused on how to improve its cultivation [55, 6, 7, 8], increase its yield [9] and use it in controlling pests or nutritional purposes [10, 11]. Little information is available on management of its waste after consumption, perhaps through composting and anaerobic fermentation.
The aim of this paper is to determine the proximate content and phytochemical screening of fluted pumpkin pod waste.
2. Materials and Method
2.1. Sample Collection
Pumpkin pod waste was obtained from Obodo Ahiara community in Ahiazu-Mbaise L. G. A of Imo State, Nigeria. The waste was pulverized using a mechanical grinder to obtain a smooth mixture.
2.2. Experimental Design
After grinding of the wastes, approximately 500g of solid waste was weighed using electronic weighing balance and used for proximate and phytochemical screening using standard analytical methods.
2.3. Proximate Analysis
Determination of Moisture content [12]
A dry clean petridish was placed in an oven at 80°C for about 30 minutes, cooled in a desiccator and weighed as (w). 5g of the samples was added to the petridish and weighed as (b). The petridish and its content were placed in an oven adjusted to 70°C. After 5 hours, the petridish containing the sample was removed and quickly transferred to a desiccator for cooling. The petridish was put back into the oven and adjusted to 105°C for another 5 hours after which it was removed, put in desiccators for cooling. This process was repeated and weighed until a constant weight (c) was obtained.
The % moisture content was determined as follows;
% moisture content = 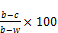 (1)
(1)
Where
W = weight of moisture can; b = weight of petridish + sample
c = weight of petridish + sample after drying
Determination of ash content [12]
An empty crucible was first ignited in a muffle furnace for 1min and allowed to cool in desiccators containing silica gel. 5g of the sample was accurately weighed into the preheated dish. The weight of the porcelain dish and the samples were noted. Afterwards, the dish was heated with a Bunsen burner in a fume cupboard until smoking ceases and later transferred into a muffle furnace at 550-570°C for about 18-24hours to burn off all organic matter. After ashing, the crucible was removed from the furnace and placed in desiccator to cool at room temperature and weighed. The percentage ash content of the sample was calculated thus;
% Ash = 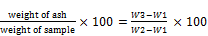 (2)
(2)
Where
W1 = weight of empty crucible; W2 = weight of crucible + sample before ashing
W3 = weight of crucible + sample after ashing
Determination of lipid [12]
A 5g of the sample was weighed into a thimble and was extracted with petroleum ether until it siphons using the Soxhlet extraction method. The lipid was exhaustively extracted using petroleum ether at 40 – 60°C for 6hrs. The sample in the thimble was removed and dried in air at 50°C for 5mins, cooled in a desiccator and weighed. The % lipid content was calculated as follows;
% Lipid = 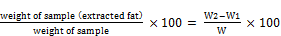 (3)
(3)
Where
W1 = weight of empty thimble; W2 = weight of thimble + sample
W = weight of sample used
Determination of crude fiber [12]
A 2g of the defatted sample was weighed into conical flask and 200mls of 1.25% of boiling sulphuric acid was added within a minute. The content of the flask was filtered through a buchner funnel prepared with wet 12.5cm filter paper. The sample was washed back into the original flask with 200mls of 1.25% NaOH, and boiled for 30mins. All insoluble matter was transferred to the crucible and treated till the sample was free from acid. The sample was again washed in a muffle furnace at 550°C/hr. The crucible was then cooled in desiccator and reweighed.
% Crude fiber = 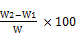 (4)
(4)
Where
W = weight of sample; W1 = weight of crucible+ sample
W2 = weight of crucible+ filter paper after ashing.
Determination of Crude protein [12]
A 1g of the sample was weighed and transferred into Khedahl flask. Few chips of antibumping granules, 4g of digestion catalyst and 20mls of conc. sulphuric acid were added at a 40°C angle with a retort stand on an electro thermal heater. The flask was gently heated for frothing to occur and subside, and then heat was increased to about 250°C. The digestion was carried out within 2-6 hours by which time the entire sample was digested completely. The digest was cooled to room temperature and diluted to 100mls with distilled water.
For distillation, 20mls aliquot of the digest was transferred into a round bottomed flask. This flask was connected to a Liebig condenser through a monoarm steel head (Adaptor). The liebig condenser was connected to a receiver flask through a receiver adapter. 10mls of 2% boric acid and two drops of double indicator were pipetted into the distillation flask. 30mls of 40% sodium hydroxide was injected into the distillation flask through a cork with the aid of a syringe. The flask was heated for 10mins to digest the content. The distillate was collected in the boric acid and then titrated with 0.1M HCL. The vol. of HCl added was recorded as the titre value. The % Crude protein was calculated thus;
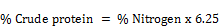 (5)
(5)
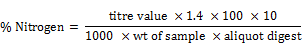
Where, 1.4 = N2 equivalent to 0.1NHCI used in titration
100 = Total volume of digest
Determination of total carbohydrate [12]
The total carbohydrate content of the sample was estimated as the Nitrogen free extract (NFE). The arithmetic different methods involve adding the total percentage value of crude volume.
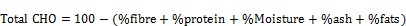 (6)
(6)
2.4. Phytochemical Screening
Preparation of the extracts
Ten grams (10g) of the sample was soaked in 100ml of water in a beaker and left for about 8hrs. The solution obtained was filtered using filter paper, and the filtrate used for phytochemical screening.
Quantitative test for phytochemicals
Determination of Saponins [12]
A 10g of the ground sample was measured into a conical flash and 100ml of 20% ethanol added to it. The suspension was heated over hot bath at 55°C for 12hrs with continuous stirring using a magnetic stirrer. The mixture was filtered and the residue re-extracted with another 200ml of 2% aqueous ethanol. The combined extract was reduced to 40ml of the original size over a water bath at about 55°C. The purification process was repeated two more times. A 4g of sodium chloride (NaCl) was added to adjust the pH meter. The solution was shaken with 60ml and 30ml portions of n-butanol extract and later washed twice with 10ml of aqueous NaCl. The remaining solution was evaporated to dryness in water bath. After evaporation, the sample was dried in the oven to a constant weight. The saponin content was calculated in g/100g as;
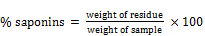 (7)
(7)
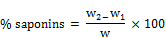
Where
W = weight of sample; W1 = weight of evaporating dish
W2 = weight of evaporating dish + saponin content after drying
Determination of Alkaloids [12]
A 2g of freshly crushed sample was weighed and dispensed into 100ml of 10% acetic acid. The mixture was shaken and allowed to stand for 4hrs before filtration. The mixture was filtered to remove all debris and evaporated to ¼ of the original volume. A 1% conc. ammonium hydroxide (NH4OH) conc. was added drop wise to precipitate the alkaloids. It was filtered with well weighed ppt. in the filter paper, oven dried at 60°C for 30mins and then reweighed. The alkaloid content was calculated as;
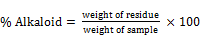 (8)
(8)
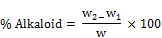
Where
W = weight of sample; W1 = weight of empty filter paper
W2 = weight of filter paper of ppt.
Determination of Flavonoids [13]
The method used for determination of flavonoids was that of Bohn and Kocipai (1994). 5g of the blended sample was extracted repeatedly and separated with 50ml of 40% aqueous methanol at room temperature. The solution was shaken for homogeneity and left to stand for about 4hrs and later filtered into a weighed beaker. The filtrate was later transferred into a crucible and evaporated to dryness over a water bath, then dried in an electric oven to a constant weight. The flavonoid content was expressed in percentage as follows;
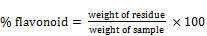 (9)
(9)
Determination of cyanogenic glycoside [12]
One gram (1g) of sample was weighed into a 250ml round bottomed flask. 200ml distilled water was added and allowed to stand for 2hrs (for autolysis to occur). Full distillation was then carried out and 150-170mls of distillate was collected in a 250ml conical flask containing 2mls of 2.5% NaOH.
Anti-foaming agent (tannic acid) was added before distillation. To 100ml of the distillate containing cyanogenic glycoside, 8ml of 6N NH4OH and 2mls of 5% potassium iodide (KI) was added, mixed and titrated with 0.02M silver nitrate (AgNO3) using a micro burette against a black background. Permanent turbidity indicated end point.
Cyanogenic glycoside in the sample was calculated thus;
 (10)
(10)
Determination of tannin [12]
A 0.5g of the sample was weighed into 100ml plastic bottle. 500mls of distilled water was added and shaken for 1hr in a mechanical shaker. Then 5mls of the filtrate was pipette out into a tube and mixed with 3mls of 0.1M Iron (III) chloride (FeCl3) in 0.1N hydrochloric acid (HCI) and 0.008M potassium ferrocyanide K4[Fe(CN)6]. The absorbance was measured in spectrophotometer at 720nm wavelength within 10mins. A blank sample was prepared, and the color also developed and read at same wavelength. A standard was prepared using tannic acid to get 100ppm and measured using the formula below.
Tannin = An/As x C x 100/W x vf/vg (11)
Where
An = Absorbance of test sample; As = Absorbance of standard solution
C = Concentration of standard solution; W = Weight of sample used
Vf = Total volume of extract; Vg = volume of extract analyzed
2.5. Method of Data Analysis
All data collected were subjected to descriptive and one way analysis of variance using Statistical Package for Social Sciences (SPSS), Inc.20.0 software. All data were represented in mean±standard deviation (M±S.D). Confident level of determination (P=0.05).
3. Result
Table 1. Proximate analysis of fluted pumpkin (Telfairia occidentalis) pod waste.
| Proximate content | Pumpkin (Telfairia occidentalis) pod waste (%) |
| Moisture | 43.18±0.59 |
| Ash content | 6.26±0.59 |
| Crude fibre | 15.43±0.01 |
| Lipid content | 7.43±0.01 |
| Crude protein | 10.73±0.20 |
| Carbohydrate | 16.97±0.21 |
Values are mean ± standard deviation of triplicate determinations (n=3).
Table 2. Qualitative results of phytochemical contents of fluted pumpkin (Telfairia occidentalis) pod waste.
| Parameters | Result |
| Tannins | ++ |
| Saponins | + |
| Alkaloids | + |
| Flavonoids | ++ |
| Cardiac glycosides | ++ |
| Oxalate | ++ |
| Phytate | + |
| Phenol | + |
Key: ++ = high; + = low
Table 3. Quantitative result of phytochemical content of fluted pumpkin (Telfairia occidentalis) pod waste.
| Phytochemical content | Pumpkin pod waste |
| Tannins | 6.41±0.95% |
| Saponins | 0.83±0.01% |
| Alkaloids | 0.66±0.01% |
| Flavonoids | 1.48±0.01% |
| Cardiac glycosides | 9.37±0.02% |
| Oxalate content | 3460.00±0.00mg/100g |
| Phytate | 0.65±0.02% |
| Phenol content | 0.63±0.00% |
Values are mean ± standard deviation (M±S.D) of triplicate determinations (n=3).
4. Discussion and Conclusion
The proximate analysis of fluted pumpkin pod waste revealed 15.43% of fibre and 16.97% carbohydrate (Table 1). The fibre content together with the cellulose contents of fluted pumpkin provides substrate for cellulose action. Also, the moisture content revealed 43.18% showing that this waste has low storage capacity. The crude fibre contents of fluted pumpkin pod waste (15.43%) were high and compared favourably with those of Lasianthera africana (15.3 – 18.1% dry mass) [14] and Heinsia crinata (13 – 15% dry mass) [15]. Although intake of dietary fibre can lower the serum cholesterol level, risk of coronary heart disease, hypertension, diabetes and colon and breast cancer, the major problem associated with nutrition of vegetables by human is the high fibre content which can cause intestinal irritation and lower bioavailability [16].
Ash contents (an index of mineral contents in biological mass), were relatively lower in fluted pumpkin pod waste (6.26%) than in fluted pumpkin (9.68%), bitter leaf, (15.86%) and Moringa oleifera (15.09%) leaves [16]. Hence, this waste could however not be a good source of mineral elements (Tables 1).
The crude protein content in fluted pumpkin pod waste (10.73%) is low when compared to 24% in Amaranthus vividis [17], 20.72% in Moringa oleifera [18], 21.0% in Lasianthera africana and 15.0% in Heinsia crinata [15, 16].
Phytochemical screenings of the pumpkin pod waste revealed tannins, saponins, alkaloids, flavonoids, cardiac glycosides, oxalate and phenol content (Tables 2 and 3). "Tannins are known to inhibit the activities of digestive enzymes and nutritional effects of tannin are mainly related to their interaction with protein. Tannins form protein complexes which are insoluble hence, decreases the digestibility of protein" [19]. The values of tannins (6.41%) reported in this study were high and could pose toxicity if used for nutritional prupose. The value was however low when compared to 13.3, 19.1 and 99.2 g/kg tannin reported for cashewnut, fluted pumpkin and raw breadnut, respectively [20]. Studies on rats, chicks and livestock revealed that high tannin in diet adversely affects digestibility of proteins and carbohydrates, thereby reducing growth, feeding efficiency, metabolizable energy and biovailability of amino acids [21].
Phytate recorded here was 0.65% (Table 3). The values reported fall within the level of phytate in Thailand fruits commonly consumed by diabetic patients; guava 0.8%, mango 0.86% and pineapple 0.90% [22]. The problem with phytic acid in foods is that it can bind some essential mineral nutrients in the digestive tract and can result in mineral deficiencies. However, the level of phytate recorded here is low and might not pose any health hazard when compared to a phytate diet of 10 – 60 mg/g if consumed over a long period of time that has been reported to decrease bioavailability of minerals in monogastric animals [23]. Phytic acid also binds to phosphorus and converts it to phytate, while other mineral elements like calcium, zinc, manganese, iron and magnesium are converted to the phytic complexes, which are indigestible substances, thereby decreasing the bioavailability of these elements for absorption. Phytic acids also have a negative effect on amino acid digestibility, thus posing problem to non-ruminant animals due to insufficient amount of intrinsic phytase necessary to hydrolyze the phytic acid complex. Its presence however, is also beneficiary because it may have a positive nutritional role as an antioxidant and anti-cancer agent [24].
Oxalate is a concern because of its negative effect on mineral availability. High oxalate diet can increase the risk of renal calcium absorption and has been implicated as a source of kidney stones [25]. Oxalate recorded in this study was 3460.00mg/100g (Table 3).
The levels of oxalate in the studied fluted pumpkin pod waste might not play important role in their nutritive values. The high concentration of some of these phytochemicals confirms fluted pumpkin pods as a waste that is toxic and harmful to life. In nutshell, it can be concluded that fluted pumpkin pod waste are moisture, fibre and tannins with low phytate, phenol and oxalate contents, hence it might not be ideal for feeding studies.
References
- Renner, S. S., Scaefer, H. and Koccyan, A. (2007). Phytogenetics of cucumis (curcurbitaceae). Journal of Food, Agriculture and Environment, 4 (1), 155-156.
- Jeffrey, C. (2005). Cucurbitaceae. In: Miline- Redhead, E. and Polhill, R. M. (Editors). Flora of tropical East Africa Crown Agents for oversea Governments and Administrators, London, United Kingdom. 157.
- Egbekan, M. K., Ada-sulelman, E. O. and Akinyeye, O. (1998). Utilization of fluted pumpkin fruit (Telfairia occidentalis) in marmalade manufacturing. Plant Foods for Human Nutrition, 52 (2), 171-176.
- Balogun, M. O., Afolabi, O. O., Kupolati, M. D. and Ajibade, S. A. (2006). Ethnobotany of Telfairia occidentalis among Igbos of Nigeria. Journal of Food, Agriculture and Environment, 4 (1), 155-156.
- Ajayi, S. A., Dulloo, M. E., Vodouhe, R. S., Berjak, P. and Kioko, J. I. (2004). Conservation status of Telfairia spp in sub-Saharan Africa. Paper presented at the Regional workshop on Plant Genetic Resources and food security in West and Central Africa, held at IITA, Ibadan, 26-30.
- Opabode, J. T. and Adebooye, O. C. (2005). Application of biotechnology for the improvement of Nigerian indigenous leaf vegetables. African Journal of Biotechnology, 4 (2), 138-142.
- Akanbi, W. B., Adebayo, T. A., Togun, O. A., Adeyeye, A. S. and Olaniran, O. A. (2007). The Use of Compost Extract as Foliar Spray Nutrient Source and Botanical Insecticide in Telfairia occidentalis. World Journal of Agricultural Sciences, 3 (5), 642-652.
- Ehiagbonare, J. E. (2008). Conservation studies on Telfairia occidentalis Hook. F. A. indigenous plant used in enthnomedicinal treatment of anemia in Nigeria. African Journal of Agricultural Research, 3 (1), 074-077.
- Odiaka, N. I. and Schippers, R. R. (2004). Telfairia occidentalis Hook. f, in G. J. H. Grubben and O. A. Denton (Editors), Plant Res. Trop. Afr. 2: Vegetables, (PROTA Foundation, Netherlands/Backhuys Publishers: Leiden, Netherlands/CTA Wageningen, Netherlands, 522-527.
- Mohammed, A. A. (2004). Chemical composition and oil characteristics of pumpkin (cucurbitamaxima) seed kernels. Food Sci. and Agric Res., 129, 518.
- Nwanguma, E. I., Akinfasoye, J. A., Aminu-Taiwo, B. R. and Akanbi, W. B. (2010). Effectiveness of intercropping and staking in the management of root-knot nematode for rural farmers in intensive mixed vegetable cropping systems in South-western Nigeria. International Journal of Organic Agriculture Research and Development, 1 (1), 25-36.
- Association of Official Analytical Chemists (1990). Method of analysis. Washington DC, U.S., 15, 1250-1255.
- Bohn, C. and Kocipai, G. (1994). Method of analysis. Washington DC, U.S., 8, 1233-1246.
- Isong, E. U. and Idiong, U. I. (1997). Comparative studies on the nutritional and toxic composition of three varieties of Lesianthera africana. Plant Foods for Human Nutrition, 51: 79-84.
- Udosen, E. O., Udok, U. E. and Unuigue, O. S. (1998). The comparison of the Nutrient composition of Lasianthera africana and Heinsia crinata. J. Food Biochem., 23, 571-576.
- Effiong, G. S., Ogban, P. I., Ibia, T. O., & Adam, A. A. (2009). Evaluation of Nutrient-supplying Potentials of Fluted Pumpkin (Telfairia occidentalis, Hook, F.) And Okra (Abelmoschus esculentus) (L.) Moench, 2 (3), 209–214.
- Sena, L. P., vander Jagt, D. J., Rivera, C., Tsin, A. T. C., Muhammadu, I., Muhamadou, O., Milson, M., Pastosyn, A. and Glew, R. H. (1998). Analysis of Nutritional components of eight famine foods of the republic of Niger. Plant Foods Human Nutr., 52, 17-30.
- Lockeett, C. T., Calvert, C. C. and Grivetti, L. E. (2000). Energy and micronutrient composition of dietary and medicinal wild plants consumed during drought. Study of rural Fulani, Northeastern Nigeria. Int. J. Food Sci. Nutr., 51, 195- 208.
- Carnovale, E., Marletta, L., Marconi, E. and Brosio, E. (1991). Nutritional and hydration properties in cowpea. In Ng NQ, Monti LM (Eds.), Cowpea genetic resources, IITA, Ibadan, pp. 111-118.
- Fagbemi, T. N., Oshodi, A. A. and Ipinmoroti, K. O. (2005). Processing Effects on Some Antinutritional Factors and In vitro Multienzyme Protein Digestibility (IVPD) of Three Tropical Seeds: Breadnut (Artocarpus altilis), Cashewnut (Anarcardium occidentale) and Fluted Pumpkin (Telfairia occidentalis). Pak. J. Nutr., 4 (4), 250-256.
- Aletor, V. A. (1993). Allelochemicals in plant food and feeding stuffs (1) Nutritional, biochemical and physiopathological aspects in animal production. J. Vet. Hum. Toxicol., 35, 57-67.
- Suree, N., Surat, K. and Akekachai, N. (2004). Phytate and Fiber Content in Thai Fruits commonly Consumed by Diabetic Patients. J. Med. Assoc. Thai., 87 (12), 1444-1446.
- Thompson, L. U. (1993). Potential health benefits and problems associated with anti nutrients in foods. Food Res. Intl., 26, 131-149.
- Turner, B. L., Paphazy, J. M., Haygarth, M. P. and Mckelvie, D. I. (2002). Inositol phosphate in the Environment. Online J. Royal Soc., 357, 469-449.
- Chai, W. and Liebman, M. (2004). Assessment of oxalate absorption from almonds and black beans with and without the use of an extrinsic label. J. Urol., 172, 953-957.

 (Peters D. E.)
(Peters D. E.) 


