Lycosome Formulation of Dark Chocolate Increases Absorption Cocoa Catechins and Augments Their Anti-Inflammatory and Antioxidant Properties
Ivan M. Petyaev1, *, Dmitry Pristenskiy1, Tatyana Bandaletova2, Natalia E. Chalyk3, Victor Klochkov3, Nigel H. Kyle1
1Lycotec Ltd, Granta Park Campus, Cambridge, United Kingdom
2DiagNodus Ltd, Babraham Research Campus, Cambridge, United Kingdom
3Institute of Cardiology, Chernyshevskogo Str, Saratov, Russia
Email address

(I. M. Petyaev)
*Corresponding author
Citation
Ivan M. Petyaev, Dmitry Pristenskiy, Tatyana Bandaletova, Natalia E. Chalyk, Victor Klochkov, Nigel H. Kyle. Lycosome Formulation of Dark Chocolate Increases Absorption Cocoa Catechins and Augments Their Anti-Inflammatory and Antioxidant Properties. American Journal of Food Science and Nutrition. Vol. 3, No. 3, 2016, pp. 37-44.
Abstract
Despite of numerous health effects a medicinal use of dark chocolate (DC) is complicated and poorly reproducible due to limited intestinal absorption and low bioavailability of chocolate bioactives. Cocoa polyphenols have low intestinal absorption rate which translates into low bioavailability. The effect of newly developed (Lycotec Ltd, Cambridge, United Kingdom) lycosome formulation of DC on pharmacokinetics and pharmacodynamics of cocoa bioactives has been studied in 15 clinically healthy Caucasian volunteers (7 men and 8 women, aged from 23 to 65 years) clinically healthy volunteers were enrolled for the double-blind cross-over trial. Coco-Lycosome chocolate was compared with traditional dark chocolate. Pharmacokinetics and biochemical parameters were assessed during the first three hours following ingestion DC specimens. It was shown that implementation of Coco-Lycosome technology increases bioavailability of chocolate catechins without affecting the level of absorption of theobromine or caffeine. It was found that lycopene could provide a level of protection to catechins in the chocolate matrix which could result in an increase in their bioavailability of up to 10 – 20 fold. The variation in this increase among participants was thought to be related to their individual metabolic limitations in processing ingested catechins. Theobromine and caffeine levels in serum were not affected. The elevated level of catechins in blood after consumption of Coco-Lycosome chocolate was accompanied by augmentation of anti-inflammatory and antioxidant properties. Therefore incorporation of lycopene in the DC matrix and subsequent formation of Coco-lycosomes increases the bioavailability of chocolate catechins and has enhanced anti-inflammatory effect. The possible benefits of this technology, which could help to create more potent catechin-rich chocolate products, are discussed in this paper.
Keywords
Chocolate Catechins, Lycopene, Lycosome, Inflammation, Pharmacokinetics
1. Introduction
There are a growing number of scientific reports revealing the numerous health benefits of cocoa-derived products. These include effects on cardiovascular health [1], cognitive and neurological functions [2], hemostasis [3] and different metabolic pathways, including carbohydrate homeostasis [4]. Raw cocoa products and dark chocolate are reported to be of most benefit to human health, while the addition of milk to cocoa-derived products impairs their favorable effects due to interference with flavanol absorption [5]. Most of the biological effects of cocoa-derived products are attributable to cocoa flavanols and alkaloids, both of which show remarkably high content in raw cocoa powder and dark chocolate [6]. The existing interest in the health benefits of cocoa products is fueled and substantiated by multiple in vivo and in vitro experiments revealing the effects of cocoa-derived substances on various signaling pathways [7], endothelial function [8] and nitric oxide production, seemingly arising from their antioxidant properties [9]. Nevertheless, the biggest obstacle to the systematic scientific analysis of the potential health benefits of cocoa products, including dark chocolate, arises from the fact that cocoa contains multiple organic compounds with discernible biological activity. Dark chocolate is at the center of current scientific interest and besides flavanols and alkaloids it contains different polyphenols and fatty acids as well as numerous peptides, amino acids and microelements. Thus it is very unlikely that a single substance responsible for the whole range of health benefits attributed to dark chocolate consumption will ever be identified. Moreover, measurable changes in the clinical and laboratory parameters of chocolate consumers emerge at a relatively high level of daily chocolate intake. Consequently some beneficial effects of dark chocolate, and hypoglycemic effects in particular, are difficult to understand from a scientific point of view owing to the significant carbohydrate content and high calorific value of dark chocolate [10, 11, 12]. Indeed, habitual consumption of any substantial amounts of dark chocolate without an adequate increase in physical activity will eventually lead to an excessive body weight and harmful changes in BMI which endanger cardiovascular perspectives for the chocolate consumers. For this reason the development of new cocoa-derived dietary products with a lower carbohydrate load becomes a necessity for any scientifically validated recommendation suggesting regular consumption of dark chocolate. Otherwise, some limit to the recommended daily intake of dark chocolate would need to be introduced. Alternatively, as cocoa flavanols have a poor intestinal absorption rate [13], the development of new technologies which could enhance bioavailability of these molecules may represent another approach to expanding the use of cocoa-based products in functional foods, health support and medical nutrition.
In this paper we report on the successful application of proprietary Lycosome technology developed and introduced into food science by Lycotec Ltd, Cambridge, United Kingdom [4] to boost absorption of chocolate catechins as well as their antioxidant and anti-inflammatory properties.
2. Materials and Methods
2.1. Study Design
This was a cross-over study for which we originally recruited 15 clinically healthy Caucasian volunteers, 7 men and 8 women, aged from 23 to 65 years. The study was conducted at the Lycotec facilities in Cambridge UK and at the Institute of Cardiology, Ministry of Health of the Russian Federation (Saratov, RF) during 2011-2012 under a protocol approved by the local Ethics Committee. All volunteers were informed about the purpose of the study and gave written consent regarding participation in the study.
At the beginning of the first stage of the study blood pressure and pulse rate were measured and a sample of venous blood taken. Each volunteer then ingested one 100 g bar of unmodified dark chocolate. After one hour their blood pressure and pulse rate were again measured and blood samples collected. This was repeated at the second and third hour following chocolate ingestion. The blood samples obtained after third hour of the study were allowed to coagulate and the serum was separated by centrifugation. Aliquots of the serum specimens were frozen at - 80˚C for further testing.
After one week of rest following the first step of the study, each volunteer was asked to ingest one 100 g bar of Coco-Lycosome chocolate. This had no difference in appearance or taste from the control chocolate bar. Blood pressure and pulse rate were measured and blood samples collected according to the same protocol used in the first stage of the study. All participants were prohibited from ingesting any cocoa-based products not related to the study starting a week from the first scheduled visit to the clinic.
In both of these two steps the control and Coco-Lycosome chocolate looked, smelled and tasted identical. Neither the persons dispensing the samples nor the volunteers were informed about which chocolate they were taking. Hence suitable conditions had so far been provided for double-blinding the trial.
2.2. Inclusion/Exclusion Criteria
Volunteer eligibility for the study was determined by the inclusion/exclusion criteria listed below.
Inclusion Criteria included: Signed informed consent, sustained resting systolic blood pressure not exceeding 120-139 mmHg, sustained resting diastolic blood pressure not exceeding 80-89 mmHg, no anti-hypertensive, lipid-lowering or any other cardio-vascular drugs, willingness and ability to comply with the protocol for the duration of the study.
Exclusion Criteria: Unwillingness to sign informed consent, inability to comply with the protocol for the duration of the study, history of MI within the 3 months preceding the study, ejection fraction (EF) < 45%, significant medical condition that would impact safety evaluations (e.g. diabetes, significantly elevated LFT, hepatitis, severe dermatitis, cancer, severe GI disease, fibromyalgia, renal failure, recent CVA (cerebrovascular accident), pancreatitis, respiratory diseases, epilepsy, etc.), compulsive alcohol abuse (> 10 drinks weekly), or regular exposure to other substances of abuse, participation in other nutritional or pharmaceutical studies, tomato or chocolate product intolerance.
2.3. Chocolate and Lycopene Products
Chocolate Control 100 g dark chocolate bars with 85% cocoa were used produced by two different manufacturers, Green & Black’s Organic (GB), UK and Vernost Kachestvu (VK) RF. Nutritional parameters, catechin, theobromine and caffeine content of these chocolate products are presented in table 1.
Coco-Lycosome is a chocolate formulation where lycopene forms reverse micelles, or a film, or a layer which can protect bioactives in chocolate micelles or crystals (Fig 1). Due to the different fat content and composition of the chocolate products, different proprietary protocols were used for the blending of specific lycopene premixes and subsequent cooling down and tempering. The total amount of lycopene in the Coco-Lycosome prepared was 30 mg in one 100 g chocolate bar.
Table 1. Nutritional information* and concentration of bioactive molecules** in chocolate products used.
| Parameters | Composition of one 100 g chocolate bar |
| Green & Black’s Organic | Vernost Kachestvu |
| Nutritional information: | | |
| Energy value | 630 kcal | 597 kcal |
| Protein | 9.2 g | 11.3 g |
| Carbohydrates | 22.2 g | 24.1 g |
| Fat | 53.5 g | 48.3 g |
| Catechins: | | |
| Catechin s | 16 mg | 14 mg |
| Epicatechin s | 53 mg | 61 mg |
| Dimer-B2 | 16 mg | 22 mg |
| Dimer-B5 | 1 mg | 3 mg |
| Trimer | 0.149 mg | 0.371 mg |
| Theobromine | 683 mg | 817 mg |
| Caffeine | 106 mg | 80 mg |
*manufacturer information, **mean of triplicate measurements
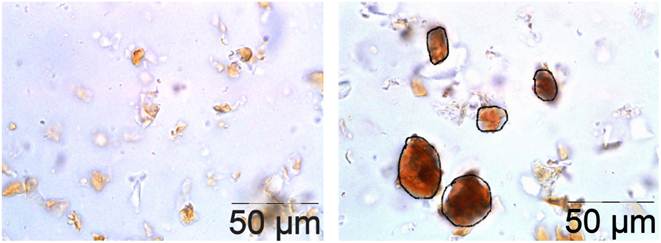
Figure 1. Control (left) and Coco-Lycosome (right) chocolate matrix.
Light microscope photo / image X100.00 OLYMPUS BX41
Coco-Lycosomes - bright orange oval bodies (circled black) contain lycopene coated inclusions
3. Methods
Biochemistry and Inflammatory Markers. C-reactive protein (CRP), were measured using commercially available analytical kits according to manufacturers’ instructions (BioSystems, Medac, R&D Systems).
Inflammatory oxidative damage (IOD). Serum samples were incubated overnight in 0.05M PBS acetate buffer (pH 5.6) to imitate the type of oxidative damage occurring during release of lysosomes following neutrophil degranulation. The following morning the reaction was terminated using trichloroacetic acid. The concentration of end products such as malonic dialdehyde (MDA) and other possible thiobarbituric acid reactive substances (TBARS) were then measured by colorimetric methods using reagents and kits from Cayman Chemical (MC, USA).
Ultra Performance Liquid Chromatography, Mass-Spectrometry (UPLC MS/MS)
Reagents and Standards Catechin, theobromine and caffeine standard materials were obtained from Fluka, as was acetic acid. Methanol (HPLC grade) and acetonitrile (HPLC grade) were obtained from Fisher Scientific (Pittsburg, PA). Water was of MilliQ grade.
Sample preparation. Serum samples (0.25 ml) were thawed at room temperature and 0.25 mL of 1% formic acid (v/v) and 1.5 mL acetonitrile were added to each sample. Samples were vortex mixed for 30 seconds and centrifuged at 18000 RCF for 15 minutes. Organic extracts were dried under vacuum for 20 minutes at 40°C. Dried extracts were then solubilized in 30 μl methanol with 200 μl 1% aqueous formic acid (v/v), vortex mixed again for 30 seconds, centrifuged at 18000 RCF and analyzed by UPLC-MS/MS.
Chocolate sample extraction. Chocolate sample extraction was performed according to the protocol published by Cooper et al [15] with some modifications. Briefly, samples were ground to obtain a homogenous material. Approximately 0.1 g of ground chocolate was treated three times with 1 ml hexane to remove fat and the residue was dried under vacuum at 40˚C. The residue was then treated three times with 1 ml of acetone/water/acetic acid (70:28:2 v/v/v) extractant using sonication and centrifugation, combining the supernatant in centrifuge tubes each time. The extracts were diluted to 30ml with Milli-Q water. After filtration using 2µm membrane filters the diluted extracts were analyzed using UPLC-MS/MS. Quantitative determination of theobromine, caffeine, (epi) catechin and its metabolites was performed based on external standards.
UPLC MS/MS Analysis. An Acquity UPLC-ESI-MS/MS system (Waters) equipped with a binary gradient pump, sample injector, column oven, degassing system and triple quadrupole mass spectrometer with an electrospray ionization (ESI) ion source driven by Waters Empower II software was used. 5µl of extract was injected into the UPLC system and separated by an Acquity UPLC HSS T3 column (1.8 µm, 2.1x75 mm) the column was maintained at 40°C. The binary system phases were (A) acetonitrile and (B) 0.1% acetic acid, with a flow rate of 0.3 mL/min. For (epi) catechin metabolite analysis of samples the 6.5 min gradient was as follows: 0.0-1.0 min, 1% A; 1.0-4.0 min, 1 - 90% A (linear); 4.0-4.9 min, 90% A; 4.9-5.0 min, 90-1% A; 5.0-6.5 min, 1% A re-equilibration. For catechin analysis of chocolate samples the 6.5 min gradient was as follows: 0.0-0.8 min, 10% A; 0.8-3.8 min, 10 - 25% A (linear); 3.8-4.0 min, 25 - 90% A (linear); 4.0-5.3 min, 90% A; 5.3 – 5.4 min, 90 - 10% A; 5.4-6.5 min, 10% A re-equilibration. For theobromine and caffeine analysis of chocolate and serum samples the 4.5 min gradient was as follows: 0.0-0.8 min, 10% A; 0.8-2.5 min, 10 – 19.9% A (linear); 2.5-2.6 min, 19.9 - 90% A (linear); 2.6-3.3 min, 90% A; 3.3 – 3.4 min, 90 - 10% A; 3.4-4.5 min, 10% A re-equilibration.
Analyses of catechins and their metabolites used the negative ion mode, analyses of theobromine and caffeine used the positive ion mode. The tuning of the mass spectrometer was optimized by infusing standards of (+/-)-catechin, theobromine and caffeine into the source. Quantitative analysis used MS/MS in the multiple reaction monitoring (MRM) ion mode (Table 2). All quantitative estimates for (epi) catechin metabolites were based on MRM peak area data expressed as (+/-)-catechin equivalents. The interassay variation was measured over the period of the analysis.
Chocolate microscopy Print smears of the surfaces of the chocolate samples were pretreated for 10 minutes at 37°C using glycerol and then studied at x100 using an Olympus BX41 microscope.
Statistics For comparison between measured parameters U-Mann-Whitney and Kruskal-Wallis tests were used. Statistical significance between two tailed parameters was considered to be p = 0.05 or less.
Table 2. Characteristics of basic metabolites.
| | RT, min | ES | [M-H]- m/z | [M+H]+ m/z | MS2, m/z | Identity | Sample |
| 1 | 1.23 | + | | 181.0 | 123.0 | Theobromine | Chocolate, serum |
| 2 | 2.46 | - | 289.0 | | 245.0 | (+/-) Catechin | Chocolate |
| 3 | 2.57 | + | | 195.0 | 138.0 | Caffeine | Chocolate, serum |
| 4 | 2.73 | - | 1153.0 | | 577.0 | Tetramer D | Chocolate |
| 5 | 2.74 | - | 577.0 | | 425.0 | Dimer B2 | Chocolate |
| 6 | 3.07 | - | 289.0 | | 245.0 | (+/-) Epicatechin | Chocolate |
| 7 | 3.21 | - | 865.0 | | 577.0 | Trimer C1 | Chocolate |
| 8 | 4.06 | - | 577.0 | | 425.0 | Dimer B5 | Chocolate |
| 9 | 2.67 | - | 465.0 | | 289.0 | (Epi) catechin-O-glucuronide | Serum |
| 10 | 2.91 | - | 369.0 | | 289.0 | (Epi) catechin-O-sulfate | Serum |
| 11 | 3.01 | - | 383.0 | | 303.0 | O-Methyl- (epi) catechin-O-sulfate | Serum |
RT- retention time
ES – electrospray ionization polarity mode
[M-H]-/ [M+H]+ - parent ion
MS2 – daughter ion
4. Results
4.1. Pharmacokinetics
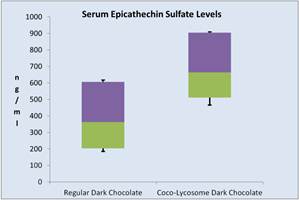
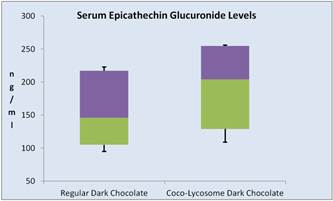
Catechin metabolites Three major (epi) catechin metabolites were identified in the serum of the volunteers following ingestion of the dark chocolate. Those were epicatechin–O-sulphate (ECS), epicatechin-O-glucuronide (ECG) and O-methyl-epicatechin-O-sulphate (OMCS). A typical serum catechin spectrum is shown in Figure 2. When the results were quantified we found that the highest concentration of the catechin metabolites was detected in the third hour following ingestion of dark chocolate. There was a distinct difference in the serum ECS concentration after ingestion of regular dark chocolate or Coco-lycosome dark chocolate (Figure 3). ECS median concentration value for Coco-lycosome chocolate was 663 ng/ml (95% CI: 904.5, 510.5) whereas corresponding value for regular dark chocolate was 363 ng/nl (95% CI: 605.5, 510.5, P=0.0038). Such an increase was observed in all participants enrolled in the study (11/11). Smaller but still statically significant increase (P=0.0264) was seen in the serum ECG levels of 9 volunteers after ingestion of Coco-lycosome chocolate with median of 204 ng/ml (95% CI: 254.5, 129) while serum ECG level after regular dark chocolate ingestion was lower (median of 146 ng/ml, 95% CI: 217, 105). Further analysis revealed that the serum epicatechin increase was independent of the age, gender, body mass, BMI and waist circumference of the study participants.
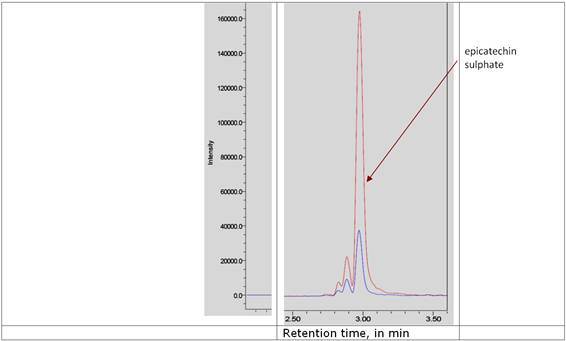
Figure 2. Typical UPLC-MS/MS analysis (m/z 369>289) of serum obtained in the cross-over study from the same individual 3 hours after ingestion of 100g dark chocolate with Coco-Lycosome (red line) or without (blue line).
A B
Figure 3. Changes in serum epicathechins after consumption of regular or coco-lycosome dark chocolate (box-and- whisker analysis).
4.2. Theobromine and Caffeine
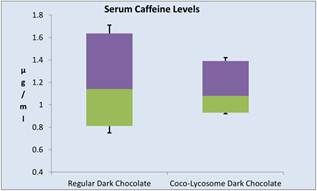
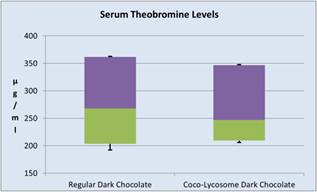
Peak serum concentration of theobromine was observed in most volunteers after 2 hours following chocolate ingestion. Peak theobromine values were slightly higher after regular dark chocolate ingestion (median serum concentration of 268 μg/ml, 95% CI: 361.5, 203.5) as compared to Coco-lycosome chocolate (median of 247 μg/ml, 95% CI: 346.5, 209.5, P=0.03662). Peak serum concentration of caffeine was seen in most volunteers after 1 hour of the post-ingestion period. However there was no statistically significant difference (P=0.4009) between two groups of the study (Figure 4).
4.3. Postprandial Oxidative Stress and Inflammation
Ingestion of 100 g of dark chocolate control resulted in a transient increase in CRP and the level of inflammatory oxidative damage (IOD) in the blood of all volunteers. These changes reached their peak at 3 hours following the beginning of the study. However, more significant changes were observed when volunteers switched to the Coco-Lycosome dark chocolate: the increase in CRP was reduced 7 fold, and the increase in IOD was not only prevented but significantly reduced to below its background baseline level.
A B
Figure 4. Changes in serum caffeine (a) and theobromine (b) after consumption of regular or coco-lycosome dark chocolate (box-and-whisker analysis).
Table 3. Effect on Postprandial Oxidative Stress and Postprandial Inflammation at 3 hours following ingestion of 100 g dark chocolate.
| Product | Changes in postprandial CRP (µg/ml) | Changes in IOD in MDA (µM) |
| Control Chocolate (n = 15) | Increased by ∆ = 0.218 + 0.038 | Increased by ∆ = 23 + 2.8 |
| Difference compared to baseline | p < 0.01* | p < 0.01* |
| Coco-Lycosome Chocolate (n = 15) | Increased by ∆ = 0.031 + 0.022 | Decreased by ∆ = - 19 + 1.6 |
| Difference compared to control chocolate | P1-3 < 0.001* | P1-3 < 0.001* |
| Difference compared to Chocolate + Lycopene capsule | P2-3 > 0.001* | P2-3 > 0.001* |
*statistically significant difference
5. Discussion
In the present paper a comparison of epicatechin pharmacokinetics following ingestion of two separate dark chocolate formulations – a standard formulation and a Coco-Lycosome formulation has been performed. In the first stage of the creation of Coco-Lycosome 5g from a standard 100g chocolate bar was melted and mixed with proprietary lycopene premix. This blend was then incorporated into the remaining 95g from the chocolate bar by melting and tempering. After ingestion of this chocolate the combined epicatechin content in the blood of three volunteers was 50% higher at its peak than when they consumed the control chocolate sample. In two of them this increase was about 100%.
If we assume that the concentration of these epicatechin metabolites in the blood is a linear function of their absorption then we may suggest that the Coco-Lycosome technology provided a significant level of protection to the molecules resulting in a 10 or even 20 fold increase in their bioavailability for individuals enrolled in the study.
However, the significant level of variability in the concentration of epicatechin metabolites in the blood, its independence from body mass and hence from the volume of circulating blood, plus its independence of gender and age in particular and hence from the possible metabolic malfunctions of an ageing liver, indicates that the function between absorption of catechins and their blood concentration is not linear. Therefore, the possible impact of Coco-Lycosome on chocolate catechin bioavailability could be even greater.
It was interesting to note that Coco-Lycosome technology helped increase catechin levels in the majority of subjects although this effect was uneven. In 4 out of 15 participants we did not observe any changes. Despite attempts to apply different protocols to the manufacture of Coco-Lycosomes for both GB and VK chocolate the pharmacokinetic response pattern remained uneven.
These results allow us to suggest that for some individuals the ingestion of 100g of dark chocolate had perhaps approached the metabolic capacity of their bodies. Any further attempts to try and deliver more of these molecules could not exceed the metabolic capacity of these individuals.
Some other individuals may have more capacity to absorb and process catechins and successful attempts to increase their delivery may result in noticeable improvements to the pharmacokinetics of these molecules. If this assumption is correct, Coco-Lycosome technology could have a more noticeable impact on the pharmacokinetics when smaller amounts of chocolate are ingested.
It was important to verify that the increase in bioavailability of epicatechins (catechins) after consumption of the Coco-Lycosome product was not accompanied by any increase in caffeine or theobromine concentrations in the blood. This apparent inconsistency can be related to the data presented here as we found that the concentration of theobromine in the chocolate used was about 8 times greater than the total amount of catechins in the chocolate and that the level of caffeine in the product was at the same level as the catechins. However, after ingestion of either Coco-Lycosome or control chocolate the concentration of caffeine in the serum was 25-50 fold higher than that of epicatechins, and the difference in theobromine was 50-150 fold. This observation indicates that caffeine and theobromine have significantly higher levels of bioavailability than catechins, and it could be that Lycosome protection is more beneficial to the catechins than to caffeine or theobromine.
Quantification of changes in the concentration of active molecules in the blood is important in the optimization and formulation of delivery technologies but the ultimate measure of the advances in any new technology is the assessment of its impact on the efficacy of the new product. The data presented here indicate that application of Coco-Lycosomes not only facilitates epicatechin delivery and absorption (and possibly other catechins as well) but may also boost the anti-inflammatory and anti-oxidative properties of chocolate.
This particular application could be of practical importance as control of postprandial inflammation and damaging oxidation is an essential part of the management of metabolic and vascular health in the growing number of consumers who regularly overeat fat-rich food where excessive consumption of chocolate may play its role.
The other potential benefit this technology may offer is a reduction in the quantity of dark chocolate used without losing its beneficial health effects. The positive implications can be numerous. Firstly, there could be a reduction in calorie intake when a smaller chocolate portion can provide the health benefit of a larger one without the ingestion of excessive cocoa butter fat mass. Secondly, the reduction or complete elimination of side effects caused by consumption of a large amount of dark chocolate which contains significant levels of theobromine, caffeine and other bioactive molecules. Smaller but more potent portions of chocolate would protect more people from these substances but still provide the health benefits of cocoa catechins. And finally, reduced consumption of cocoa for the production of functional chocolate products could significantly improve their affordability whilst retaining their health value and may boost sustainability of the whole chocolate industry.
6. Conclusion
Coco-lycosome formulation of dark chocolate increases bioavailability of cocoa polyphenols and enhances their anti-inflammatory in healthy volunteers without affecting intestinal absorption of theobromine and caffeine.
References
- Kerimi A, Williamson G. The cardiovascular benefits of dark chocolate. Vascul Pharmacol. 2015 Aug; 71: 11-5
- Katz DL, Doughty K, Ali A. Cocoa and chocolate in human health and disease.Antioxid Redox Signal. 2011 Nov 15; 15 (10):2779-811.
- Şentürk T, Günay Ş. The mysterious light of dark chocolate. Turk Kardiyol Dern Ars. 2015 Mar; 43 (2): 199-207.
- Sarriá B, Mateos R, Sierra-Cinos JL, Goya L, García-Diz L, Bravo L. Hypotensive, hypoglycaemic and antioxidant effects of consuming a cocoa product in moderately hypercholesterolemic humans. Food Funct. 2012 Aug; 3 (8): 867-74.
- Gossai D, Lau-Cam CA. Assessment of the effect of type of dairy product and of chocolate matrix on the oral absorption of monomericchocolate flavanols in a small animal model. Pharmazie. 2009 Mar; 64 (3): 202-9.
- McShea A, Ramiro-Puig E, Munro SB, Casadesus G, Castell M, Smith MA. Clinical benefit and preservation of flavonols in dark chocolate manufacturing. Nutr Rev. 2008 Nov; 66(11): 630-41.
- Ramirez-Sanchez I, Aguilar H, Ceballos G, Villarreal F.(-)-Epicatechin-induced calcium independent eNOS activation: roles of HSP90 and AKT. Mol Cell Biochem. 2012 Nov; 370 (1-2): 141-50.
- Grassi D, Desideri G, Necozione S, Ruggieri F, Blumberg JB, Stornello M, Ferri C. Protective effects of flavanol-rich dark chocolate on endothelial function and wave reflection during acute hyperglycemia. Hypertension. 2012 Sep; 60(3): 827-32.
- Gülçin İ. Antioxidant activity of food constituents: an overview. Arch Toxicol. 2012 Mar; 86(3): 345-91.
- Koegler H. Too sweet to be real? Arch Intern Med. 2012 Sep 10; 172(16): 1270-79.
- Shiina Y, Funabashi N, Lee K, Murayama T, Nakamura K, Wakatsuki Y, Daimon M, Komuro I. Acute effect of oral flavonoid-rich dark chocolate intake on coronary circulation, as compared with non-flavonoid white chocolate, by transthoracic Doppler echocardiography in healthy adults. Int J Cardiol. 2009 Jan 24; 131(3): 424-9.
- Flammer AJ, Sudano I, Wolfrum M, Thomas R, Enseleit F, Périat D, Kaiser P, Hirt A, Hermann M, Serafini M, Lévêques A, Lüscher TF, Ruschitzka F, Noll G, Corti R. Cardiovascular effects of flavanol-rich chocolate in patients with heart failure. Eur Heart J. 2012 Sep; 33 (17): 2172-80. doi: 10.1093/eurheartj/ehr448. Epub 2011 Dec 15.
- Donovan JL, Crespy V, Oliveira M, Cooper KA, Gibson BB, Williamson G. (+)-Catechin is more bioavailable than (-)-catechin: relevance to the bioavailability of catechin from cocoa. Free Radic Res. 2006 Oct; 40(10): 1029-34.
- CAROTENOID PARTICLES AND USES THEREOF. GB Patent Application No. 1101669.8, PCT/GB2012/000075, 25.01.2012.
- Cooper KA, Campos-Giménez E, Jiménez Alvarez D, Nagy K, Donovan JL, Williamson G. Rapid reversed phase ultra-performance liquid chromatography analysis of the major cocoa polyphenols and inter-relationships of their concentrations in chocolate. Agric Food Chem. 2007 Apr 18; 55(8): 2841-7.

 (I. M. Petyaev)
(I. M. Petyaev) 







