Ameliorative Effects of Some Selected Antioxidant Supplements on Aluminium Induced Nephrotoxicity of Male Wistar Rats
Onyegeme-Okerenta B. M.*, Anacletus F. C.
Department of Biochemistry, Faculty of Science, University of Port Harcourt, Choba, Rivers State, Nigeria
Email address

(Onyegeme-Okerenta B. M.)

(Onyegeme-Okerenta B. M.)
*Corresponding author
Citation
Onyegeme-Okerenta B. M., Anacletus F. C. Ameliorative Effects of Some Selected Antioxidant Supplements on Aluminium Induced Nephrotoxicity of Male Wistar Rats. Health Sciences Research. Vol. 3, No. 2, 2016, pp. 17-22.
Abstract
The effectiveness of selected antioxidants supplements (zinc, selenium, ginseng, vitamin A, vitamin C, and vitamin E) in ameliorating the toxic effect of Aluminium on the kidney of male wistar rats was investigated. Forty eight male wistar rats (100-175g) were divided into eight equal groups of six rats each and the mean weight for each group was taken. Normal drinking water and feed was given to the control group, while the other seven groups 2- 8 received 200mg/kg b.w of aluminium (Al) daily. Group 2 received only Al. Groups 3 - 8 were administered orally with zinc, selenium, ginseng, vitamin A, vitamin C, vitamin E respectively at doses 14.8mg/kg b.w, 100mg/kg b.w, 10mg/kg b.w, 100mg/kg b.w, 100mg/kg b.w, 100mg/kg b.w for 6 weeks. Administration of Al showed a significant increase (p<0.05) in the creatinine, urea and electrolyte levels, while administration of zinc, selenium, ginseng, vitamin A, vitamin C, and vitamin E ameliorated the toxic effect of aluminium when compared to the control value. Histopathological investigation of kidney of rats in the control group showed a normal architecture of kidney cells whereas the administration of Aluminium caused distortion of the renal tubular cells. Investigation of Aluminium + Antioxidant groups; the Al+Zinc, Al+Ginseng, Al+Vit A, Al+Vit C, Al+Vit E showed no obvious histological changes.However, the group treated with selenium showed a mild tubular distortion, this shows that selenium was not able to completely ameliorate the toxic effect of aluminium. This investigation showed that zinc, ginseng, vitamin A, vitamin C, and vitamin E have beneficial influences and are able to ameliorate aluminium toxicity in the kidney of male wistar rats.
Keywords
Aluminium, Antioxidants, Creatinine, Electrolyte, Nephrotoxicity, Urea, Wistar Rats
1. Introduction
Aluminium toxicity is caused as a result of excess intake of aluminium through several ways, in water, food, drugs etc. Toxic effects of aluminium depend on the amount of metal ingested, entry route, tissue distribution, concentration achieved, and excretion rate [1], [2], [3], [4]. Fatty acids common in food may facilitate the paracellular intestinal absorption of aluminium [5]. Mechanisms of aluminium toxicity include inhibition of enzyme activity and protein synthesis, alterations in nucleic acid function, and changes in cell membrane permeability. Aluminium toxicity is usually found in patients with impaired renal function. Acute intoxication is extremely rare; however, in persons in whom aluminium clearance is impaired, it can be a significant source of pathology [6]. Aluminium toxicity was originally described in the mid-to-late 1970s in a series of patients in Newcastle, England, through an associated osteomalacic dialysis osteodystrophy that appeared to reverse itself upon changing of the dialysate water to deionized water (i.e., aluminium-depleted water) [7].
Approximately 95% of the aluminium that is absorbed is then bound in the circulation by the plasma proteins albumin and transferrin, from which it is rapidly eliminated through the kidney. In healthy subjects, only 0.3% of orally administered aluminium is absorbed via the gastrointestinal (GI) tract, and the kidneys effectively eliminate aluminium from the human body. Only when the GI barrier is bypassed, such as by intravenous infusion or in the presence of advanced renal dysfunction, does aluminium have the potential to accumulate. As an example, with intravenously infused aluminium, 40% is retained in adults and up to 75% is retained in neonates [8]. Aluminium is absorbed from the GI tract in the form of oral phosphate-binding agents (aluminium hydroxide), parenterally via immunizations, via dialysate on patients on dialysis or total parenteral nutrition (TPN) contamination, via the urinary mucosa through bladder irrigation, and transdermally in antiperspirants. Lactate, citrate, and ascorbate all facilitate GI absorption. If a significant aluminium load exceeds the body's excretory capacity, the excess is deposited in various tissues, including bone, brain, liver, heart, spleen, and muscle. This accumulation causes morbidity and mortality through various mechanisms [9]. A 10-fold increase in aluminium concentrations was reported in patients with aluminium intoxication through the use of hemodialysis solutions with high levels of aluminium [7].
A possible etiologic link between aluminium exposure and Alzheimer disease emerged from a 1965 study showing that aluminium causes neurofibrillary tangles in the brains of rabbits. Subsequent research has largely failed to support this hypothesis, however. For example, the clinical manifestations and underlying neuropathology of aluminium-induced encephalopathy in dialysis patients bear no resemblance to those of Alzheimer disease [10].
Kidneys are essential to the urinary system and also serve homeostatic functions such as the regulation of electrolytes, maintenance of acid–base balance, and regulation of blood pressure (via maintaining the salt and water balance). They serve the body as a natural filter of the blood, and remove water-soluble wastes which are diverted to the bladder [11]. Insufficient levels of antioxidants, or inhibition of the antioxidant enzymes, cause oxidative stress and may damage or kill cells. Oxidative stress is damage to cell structure and cell function by overly reactive oxygen-containing molecules and chronic excessive inflammation. Oxidative stress seems to play a significant role in many human diseases; including cancers. Antioxidants are protective substances that have been investigated for their potential to help in treating patients with kidney disease [12]. Antioxidants derived from the diet or administered by researchers have been demonstrated to slow the progression of kidney disease in various animal models. The amount of protection provided by any one antioxidant will also depend on its concentration, its reactivity towards the particular reactive oxygen species being considered, and the status of the antioxidants with which it interacts [13]. This study was therefore designed to investigate the effectiveness of selected antioxidants supplements (zinc, selenium, ginseng, vitamin A, vitamin C, and vitamin E) in ameliorating the toxic effect of Aluminium on the kidney of male wistar rats.
2. Methodology
2.1. Collection of the Animals, Antioxidants Supplements and Reagents
Male wistar rats were gotten from the animal farm of the Department of Animal and Environmental Biotechnology, University of Port Harcourt Choba, Rivers State. The zinc (50mg per tablet) and ginseng (1000mg per capsule) supplement used for this research were bought from Chux medical laboratory and were manufactured by Mason vitamins Incorporation in the United States of America. Selenium (50mg per tablet) and Vitamin E (450mg per capsule) supplement was manufactured by Archy pharmaceuticals in the United States of America. Vitamin A was manufactured by Softgel Health Care Private Limited and each tablet contain USP 2000000IU. Vitamin C used was manufactured by Emzor Pharmaceuticals in Nigeria and each tablet weighed 50mg. Aluminium used to induce toxicity was obtained from the Research Laboratory of the Department of Biochemistry, University of Port Harcourt, Choba. All other reagents used were of analytical grade.
2.2. Experimental Design
A total of 48 wistar rats were obtained from the animal house of the Department of Animal and Environmental Biotechnology, University of Port Harcourt, Rivers State, Nigeria. They were housed in separate plastic cages and acclimatized for twenty-one days and feed on conventional rat feed and water. The rats were completely randomized into eight groups of six rats each; (control, aluminium, selenium, zinc, ginseng, vitamin A, vitamin C and vitamin E). Rats in the control group (Cn) were given only their food and distilled water, the aluminium group (Al) was given 200mg/kg of aluminium alongside their feed. The other groups which include the selenium (Al+Se), zinc (Al+Zn), ginseng (Al+Gn), vitamin A (Al+Vit A), vitamin C (Al+Vit C) and vitamin E (Al+ Vit E) were given 200mg/kg of aluminium as well as 100mg/kg, 14.8mg/kg, 10mglkg, 100mg/kg, 100mng/kg and 100mg/kg of the various dosages of antioxidants respectively. At the end of nine weeks of administration, rats from each group were selected randomly and sacrificed by anaesthetizing with chloroform in a box and their blood taken for analysis. Kidney function tests assayed for include creatinine, urea and electrolyte (sodium and potassium). Kidney tissue from each animal was rapidly fixed in buffered neutral 10% formalin and subjected to standard routine histological procedures as described by [14].
3. Determination of Biochemical Parameters
3.1. Determination of Urea
Urea is hydrolysed in the presence of water and urease to produce Ammonia and carbon dioxide. In a modified Berthelot reaction the ammonium ions react with hypochlorite and salicylate to form a green dye. The absorbance increase at 578 nm is proportional to the urea concentration in the sample. Reagent one was made up of 120mmol/L Phosphate buffer (pH 7.0), 60mmol/L Sodium salicylate, 5mmol/L Sodium nitruprusside and 1 mmol/L EDTA was constituted before the test. Ten micro litres of plasma and standard were added in well labelled test tubes. One thousand micro litres of reagent 1a (1ml of Urease enzyme + 100ml of reagent one was added). This was well mixed and incubated for 3minutes at 37°C. One thousand micro litres of reagent two (Phosphate buffer (pH < 13) 120mmol/L, Hypochlorite 0.6g/l Cl, Urease enzyme, urea standard, sodium azide 0.095%) was added, mixed and incubated at 37°C. Absorbance was measured within 60minutes at 578nm against a reagent blank [15], [16].
3.2. Determination of Creatinine
Creatinine in an alkaline solution forms an orange-red coloured complex with picric acid. The absorbance of this complex is proportional to the creatinine concentration in the sample. One thousand micro litres of plasma, creatinine standard and distilled water were added in well labelled test tubes. 1000μl of 10% Trichloroacetic acid was added to deproteinize the plasma. This was carefully mixed centrifuged at 4000g for 10minutes. One thousand micro litres of the deproteinized serum supernatant, creatinine standard and water were carefully collected into clean labelled respective test tubes and 1000μl Reagent 1 (equal volume of Picric acid 26mmol/l and Sodium hydroxide 1.6mmol/l) was added. This was well mixed and incubated for 20minutes at 25°C. Absorbance was measured at 546nm against a reagent blank [15], [16].
3.3. Determination of Sodium (Na+) and Potassium (K+)
A 1:5 dilution of the sample (plasma) was made with distilled water and introduced into the flame photometer. The electrical signals of the photo detector was amplified and displayed on the digital readout. The absorbance of sample is directly proportional to the concentration of electrolyte present; this was multiplied by the dilution factor to get the actual concentration of the test sample.
3.4. Statistical Analysis
In this study, data was analysed by one way ANOVA according to SPSS, version 21 program to find the means for all treatments. These means were compared using Turkey HSD and Bonferroni at 0.05 confidence limit (P < 0.05) in multiple comparison.
4. Result
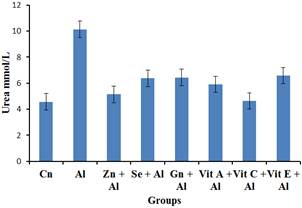
Figure 1. The impact of aluminium toxicity and the ameliorative effect of selected antioxidants on urea level of male wistar rats.
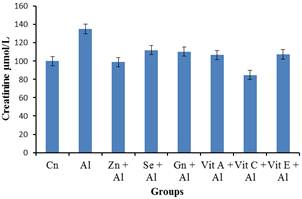
Figure 2. The impact of aluminium toxicity and the ameliorative effect of the selected antioxidants on creatinine level of male wistar rats.
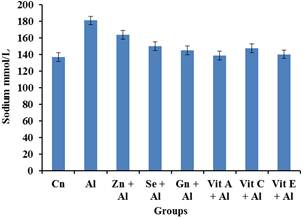
Figure 3. The impact of aluminium toxicity and the ameliorative effect of selected antioxidants on Sodium level of male wistar rats.
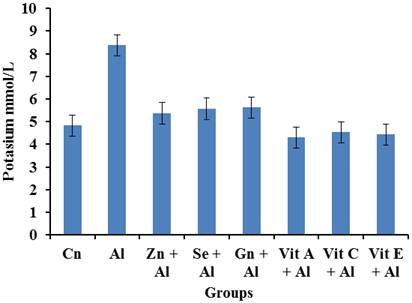
Figure 3 shows that there was an increase in the Sodium levels of the aluminium group. However, it was observed that there was a significant decrease (p<0.05) in the sodium level of the Al+Gn, Al+Vit A, Al+Vit C, Al+Vit E groups.
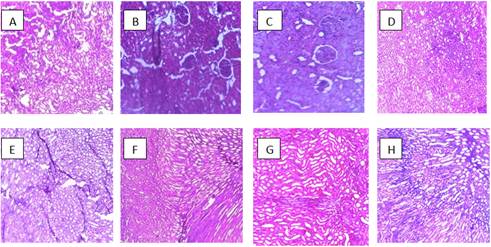
Plate 1 is photomicrographs showing the impact of aluminium toxicity and the ameliorative effect of some selected antioxidants on the kidney of wistar rats.
5. Discussion and Conclusion
The present study was carried out to evaluate the effectiveness of selected antioxidants (zinc, selenium, ginseng, vitamin A, vitamin C, and vitamin E) in ameliorating the toxicity of Aluminium on the kidney of male wistar rats. Results of electrolyte obtained showed increased levels of sodium and potassium in the group treated with aluminium when compared to the control group. However, groups treated with aluminium and selected antioxidants (selenium, zinc, Vitamin A, Vitamin C, Vitamin E and ginseng) showed a significant decrease (p<0.05) in the sodium and potassium level when compared with the aluminium treated group. Since the kidney is the seat of excretion, increased levels of sodium and potassium may suggest nephrotic toxicity by aluminium. These results were in agreement with [17] and [18] who reported the nephrotoxic effects of aluminium exposure in separate studies [19]. Has reported that exposure to heavy metals is potentially harmful. The kidney is the first target organ of heavy metal toxicity, this is because of its ability to reabsorb and accumulate divalent metals. The extent of renal damage by heavy metals depends on the nature, the dose, route and duration of exposure. Both acute and chronic intoxication have been demonstrated to cause nephropathies, with various levels of severity ranging from tubular dysfunctions like acquired Fanconi syndrome to severe renal failure leading occasionally to death.
Results of urea showed that there was a significant increase (p< 0.05) in urea level of group treated with aluminium when compared to the control group while the groups treated with aluminium + vitamin C and aluminium + vitamin E were able to ameliorate the nephrotic effects of aluminium on the urea level when compared to the control group. According to [20], increased levels of urea may indicate kidney disease. On the other hand, aluminium + Selenum, Gensing, Zinc and Vitamin A respectively, did not show obvious ameliorative effect.
Creatinine concentration of groups treated with aluminium showed a significant increase (p<0.05) in the groups treated with aluminium when compared to the control group. However, a significant decrease (p<0.05) in creatinine concentration was observed in the aluminium + antioxidants groups (aluminium + Selenum, aluminium + vitamin C, and aluminium + vitamin E) when these groups were compared with the aluminium treated group. There was no significant reduction in creatinine level observed in groups treated with aluminium + Zinc, aluminium + Gensing, aluminium + Vitamin A) when compared with the aluminium treated group. [21] reportedthat creatinine is a more accurate marker of kidney disease than urea. High creatinine level implied that many waste products in the wistar rats bloodstream would not be cleared, indicating that the kidneys were not functioning properly. This suggests that these antioxidants can remedy the toxicity effect of aluminium and return the increased level of creatinine to the control levels.
Results obtained from photomicrograph showed normal architecture in the control groups while the Aluminium and Al+Se treated groups showed distortion of the renal tubular cells. However, photomicrographs of groups treated with Aluminium + antioxidant; Al+Zinc, Al+Gn, Al+Vit A, Al+Vit C, Al+Vit E showed no obvious histologic change. This implies that selenium was not able to ameliorate the effect of aluminium on the renal tubules. This study shows that aluminium has a toxic effect on the renal tubules of the kidney. [22] and [23], observed well marked dose dependant morphological changes in the proximal tubules of the kidney and vacuolation of both the proximal and distal convoluted tubules and glomerular atrophy respectively after they administered aluminium chloride. According to [22], the individual cells of the tubules looked swollen, tubular dilatation along with atrophy. In the present study the dose administered was quite high and given through oral route and therefore changes were found even under a light microscopic examination. It can be concluded that the administration of antioxidants has a therapeutic role in preventing aluminium-induced nephrotoxicity, possibly through its unique cytoprotective properties.
The elevation in electrolyte levels, urea and creatinine in Al treated rats can be considered as a significant marker of renal dysfunction. This fact was supported by [24], who reported that Al intoxication intensifies acid-secretory function of kidney and changes the transport of sodium. Similarly, [25] reported that Al has been implicated in the pathogenesis of several clinical disorders, such as renal dysfunction. Al has adverse effects on human health as well as animals. The results demonstrated that the administration of Aluminium to wistar male rats at a dose of 200mg/kg daily for a period of 6 weeks is capable of inducing marked alterations in kidney function. The use of some selected antioxidants ameliorated the toxic effects of Aluminium on the kidney.
Acknowledgement
Management and Staff of Healthwise Diagnostic Laboratory, Rumuomasi, Port Harcourt, Rivers State, Nigeria.
References
- Riihimaki, V., Valkonen, S., Engstrom, B., Tossavainen, A., Mutanen, P. & Aitio, A. (2008). Behavior of aluminium in aluminium welders and manufacturers of aluminium sulphate-impact on biological monitoring. Scandinavian Journal of Work Environmental Health. 34(6):451-62.
- Vasudevaraju, P., Govindaraju, M., Palanisamy, A. P., Sambamurti, K. & Rao, K. S. (2008). Molecular toxicity of aluminium in relation to neurodegeneration. Indian Journal of Medical Research. 128(4):545-56.
- Lemire, J., Mailloux, R., Puiseux-Dao, S. & Appanna, V. D. (2009). Aluminium-induced defective mitochondrial metabolism perturbs cytoskeletal dynamics in human astrocytoma cells. Journal of Neuroscience Research. 87(6):1474-83.
- Hernandez, G., Bollini, A. & Huarte, M. (2008). In vitro effect of aluminium upon erythrocyte membrane properties. Clinical Hemorheology and Microcirculation. 40(3):191-205.
- Aspenstrom-Fagerlund, B., Sundstrom, B., Tallkvist, J., Ilback, N. G. & Glynn, A. W. (2009). Fatty acids increase paracellular absorption of aluminium across Caco-2 cell monolayers. Chemico- Biological Interactions, 181(2), pp 272-278.
- Utpal, K. S., Layland, F. C & Prasad, R. (2013). Poisoning in children.4th edition, Jaypee Brothers Medical Publishers (P) Limited. New Delhi p 75.
- Alfrey, A. C., LeGendre, G. R. & Kaehny, W. D. (1976). The dialysis encephalopathy syndrome. Possible aluminium intoxication. New England Journal of Medicine. 294(4):184-8.
- Brown. R. O., Morgan, L. M., Bhattacharya, S. K., Johnson, P. L., Minard, G. & Dickerson, R. N. (2008). Potential aluminium exposure from parenteral nutrition in patients with acute kidney injury. Annals of Pharmacotherapy. 42(10):1410-5.
- Verstraeten, S. V., Aimo, L. & Oteiza, P. I. (2008). Aluminium and lead: molecular mechanisms of brain toxicity. Archive Toxicology. 82(11):789-802.
- Lidsky, T. I. (2014). Is the Aluminium Hypothesis dead? Journal of Occupational and Environmental Medicine. 56(5): 73-9.
- Ingrid Fredrikson. (2015). The journey to life or death. Strategic Book Publishing and Rights Co., LLC USA/Singapore, p 130.
- Hamid, A. A., Aiyelaagbe, O. O., Usman, L. A., Ameen, O. M. & Lawal, A. (2010). Antioxidants: Its medicinal and pharmacological applications. African Journal of Pure and Applied Chemistry, 4(8):142-151.
- Vertuani, S., Angusti, A. & Manfredini, S. (2004). The antioxidants and pro-antioxidants network: an overview. Current Pharmaceutical Design.10 (14): 1677–94.
- Brown, H. S., 2002. "Hematoxylin and eosin (the routine stain). H & H informational primer." Sigma-Aldrich Corporation, pp. 1–3.
- Tobacco, A., Meiattini F., Moda E.&Tarli P. (1979). Enzymatic colorimetric test for Urea. Clinical chemistry. 25, 336.
- Tietz, N. W. (2000). Fundamentals of Clinical Chemistry. 6th Ed. Philadelphia W.B. Saunders co. pp 744-745, 788-789.
- Steenland, K., K. Rosenman, E. Socie & Valiante, D. (2002).Silicosis and end-stage renal disease. Scandinavian Journal of Work, Environmental and Health, 28: 439-442.
- Akinola, M.O., N.A. Okwok & T. Yahaya, (2008). The effects of cement dust on albino rats (Rattus norvegicus) around West African portland cement factory in Sagamu, Ogun state, Nigeria. Res. J. Environ. Toxicology, 2:1-8.
- Barbier, O., Jacquillet, G., Tauc, M., Cougnon, M. & Poujeol, P. (2005). Nephron physiology. 99(4): 105-10.
- Ajeniyi, S. A. & Solomon, R. J. (2014). Urea and creatinine of Clarias gariepinus in three different commercial ponds. Nature and Science, 12(10): 124-138.
- National Kidney Foundation, (2002). K/DOQI clinical practice guidelines for chronic kidney disease: Evaluation, classification and stratification. American Journal of Kidney Diseases, 39: S1-S266.
- Somova, L. I., Missankov, A. & Khan, M. S. (1997) Chronic Aluminium intoxication in rats: Dose-dependent Morphological changes. Methodology &findings. Experimental Clinical Pharmacology. 19(9): 599-604.
- Buraimoh, A. A. & Ojo, S. A. (2012). Effects of Aluminium Chloride Exposure on the Histology of Kidney of Wistar Rats. International Journal of Biology, Pharmacy and Allied Sciences, 1(11):1556-1568.
- Rudenko, S. S., Bodnar, B. M., Kukharchuk, O. L., Mahalias, V. M., Rybshchka, M. M., Ozerova, I. O., Chala, K. M & Khalaturnik, M. V. (1998). Effect of selenium on the functional state of white rat kidney in aluminium cadmium poisoning. Ukrainskii Biokhimicheskii Zhurnal. 70:98-105.
- Katyal, R., Desigan, B., Sodhi, C. P & Ojha, S. (1997). Oral aluminium administration and oxidative injury. Biological Trace Element. Research. 57:125-130.

 (Onyegeme-Okerenta B. M.)
(Onyegeme-Okerenta B. M.)  (Onyegeme-Okerenta B. M.)
(Onyegeme-Okerenta B. M.)