Argon and Krypton Adsorption Studies on the Single Walled Carbon Nanotube
A. Diama1, 2, *, Zoro Diama E. G.1, M. Grafoute1, V. Manu3, F. J. Guehi1, A. F. Yebouet1, H. C. Bajaj3
1Physics Department, Condensed Matter and Technology Laboratory, Felix Houphouet Boigny University of Cocody, Abidjan, Côte d’Ivoire
2Science Technology Engineering Mathematics Department, International University of Grand-Bassam, Bassam, Côte d’Ivoire
3Discipline of Inorganic Materials and Catalysis (DIMC), CSMCRI, Bhavnagar, India
Email address

(A. Diama)
*Corresponding author
Citation
A. Diama, Zoro Diama E. G., M. Grafoute, V. Manu, F. J. Guehi, A. F. Yebouet, H. C. Bajaj. Argon and Krypton Adsorption Studies on the Single Walled Carbon Nanotube. American Journal of Materials Research. Vol. 3, No. 3, 2016, pp. 15-20.
Abstract
This paper studied the adsorption of argon and krypton onto Single Walled Carbon Nanotubes (SWCNTs) at liquid nitrogen temperature (77 K). The volumetric method was used to characterize our sample after purification by nitrogen adsorption. This method was also used to examine the effect of the size of molecules on the adsorption properties (energy and adsorption capacity) of SWCNTs and determined the adsorption sites. The results obtained show that the specific surface of the sample almost doubled after purification. This research showed that the energy and the adsorption capacity decrease when the molecule size increases; small molecules have access to all the adsorption sites of SWCNT and those of larger size do not adsorb in the lower interstitial channels. Finally, the studies have shown that the krypton does not adsorb in the micropores.
Keywords
Adsorption, Krypton, Argon, SWCNT, Adsorption Energy, Adsorption Capacity
1. Introduction
Discovered in 1991 by Sumio Iijima [1], carbon nanotubes (CNT) are a tubular form of carbon with a diameter of one nanometer (≈1nm) and a length of several microns. CNTs are graphene sheets (made of carbon atoms arranged in a hexagonal array) wound on themselves. There are two types of nanotubes: The Single-Walled Carbon Nanotube (SWCNT) corresponding to a single tube of a graphene sheet rolled on itself and the Multi-Walled Carbon Nanotube (MWCNT) consisting of several graphene sheets wrapped around each other or a single graphene sheet rolled several times on itself. The graphene sheets can wrap in different ways leading to different structures. Carbon nanotubes are defined by three parameters: diameter, length and angle of helicity (θ) that depends on the orientation of the hexagonal patterns with respect to the tube axis (winding type).
The winding of the graphene sheet may be represented by a vector.
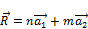 (1)
(1)
n and m are integers called Hamada indices [2]; 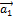 and
and 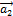 are two basis vectors of the graphene network forming between them an angle of 60°.
are two basis vectors of the graphene network forming between them an angle of 60°.
Unlike diamond, no natural nanotubes have been discovered to date. CNT synthesis is an active research topic since their discovery [3,4]. The most used syntheses can be classified into two major groups: the high-temperature synthesis (Arc ablation and Laser ablation) and syntheses at medium temperature CVD (Chemical Vapor Deposition) in the presence of catalysts. For large scale production of CNTs, the CVD method is the most used.
Impurities found in the nanotubes are of two types: metal particles from catalysts (Co, Ni) and carboniferous species such as amorphous carbon, fullerenes and nanocrystalline graphite. The purification of CNTs is an important issue which is the subject of numerous publications [5,6]. Main purification methods are: chemical methods (gas-phase oxidation, liquid phase oxidation or acid treatment) and physical methods (centrifugation, filtration, ultrasonication, magnetic).
In the case of our study, acid treatment was used to purify the SWCNTs [7] and post-synthesis treatment (purification) to optimize the properties of nanotubes for potential applications (water purification, gas storage, transistors, ultra miniaturized chips.). Carbon nanotubes have excellent properties (mechanical, electrical, adsorption) that allow for many applications: Those include the storage of gas, especially the storage of hydrogen.
The storage of hydrogen has been the subject of many studies. In the 1980’s, studies were conducted on activated carbon for hydrogen storage [8-10]. Despite the good adsorption properties of activated carbon due to its large surface area, the results were not promising. Activated carbon was ineffective for hydrogen storage with a lower storage capacity to 1% by mass, far from the requirements of the US Department of Energy (DOE) (6.5% by mass is 62 kg /m3 at room temperature). It was in 1997 that the first experimental study on the storage of hydrogen by carbon nanotubes, especially by SWCNTs, was performed [11]. The results obtained are better; the hydrogen storage capacity is between 5% and 10% by mass.
Since the first study on the storage of hydrogen, the adsorption properties of carbon nanotubes have aroused much interest when storing, detecting and separating gasses [12].
Unfortunately, those properties have not yet been used successfully, mainly because of impurities in the CNTs. The morphology of the adsorbed gas can also prove to be a significant factor for the storage of gasses.
This comparative study of the adsorption of argon and krypton onto SWCNTs aims to provide answers to the gas storage mechanism through the use of adsorption properties of SWCNTs.
2. Experimental Section
2.1. Adsorption Phenomenon
When a gas is brought into contact with a solid, a portion of the gas molecules simply attach to the solid surface, a phenomenon called adsorption. Adsorption is the "sticking" of molecules to the solid surface. The solid is called the substrate or adsorbent and the adsorbed molecules are called adsorbates. Figure 1 shows a multilayer adsorption of molecules model.
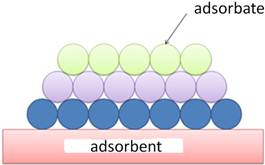
Figure 1. Multilayer adsorption of molecules model.
There are two types of adsorption characterized by the nature of the bonds between the substrate and the adsorbed molecules. Physical adsorption or physisorption is due to Van der Waals forces and chemical adsorption or chemisorption is due to electron transfer between the solid and gas (ionic bonds).
Physisorption is a reversible phenomenon in which the adsorbed molecules can be desorbed by a slight increase in temperature or decrease in pressure due to the Van der Waals force being very low at ordinary pressures in gases. On the contrary, the desorption of chemisorbed molecules is difficult since ionic bonds are very energetic and this process is sometimes irreversible.
2.2. Adsorption Sites
During adsorption, the gas molecules are hosted by sites on the sample surface called adsorption sites. The sample used in this study (SWCNT) has four adsorption sites [13] (Figure 2):
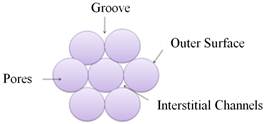
Figure 2. Adsorption sites on the SWCNT.
1. The outer surface of the tubes.
2. Grooves: space between two adjacent tubes.
3. interstitial channels (IC): space between tubes.
4. Pores.
Tube bundles are composed of SWCNTs of varying diameters. The inhomogeneity of those diameters means that ICs are of different sizes; we can find wide and narrow ICs [14].
The pores are classified according to their size into three groups (Figure 3) according to IUPAC (International Union for Pure and Applied Chemistry)
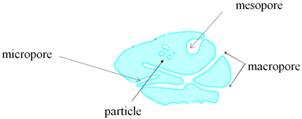
Figure 3. Different types of pores.
1. micropores: d < 2nm
2. mesopores 2 nm < d < 50 nm
3. macropores: d > 50 nm
Where d is the mean pore diameter.
2.3. Experimental Determination of the Adsorption Energy (Eads) and the Adsorption Capacity (Cads) of the Gas
The adsorption energy is the energy of substrate - adsorbate interaction added to the energy of adsorbate-adsorbate interaction. Using the BET Equation 2:
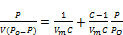 (2)
(2)
V = Amount of nitrogen adsorbed at a relative pressure P / P0
Vm = Capacity of the monolayer, the volume of gas adsorbed.
C = Constant
The Eads adsorption energy is given in Equation 3:
Eads = RT logC (3)
C: determined from the slope constant 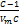
R: gas constant (8.31 J / (mol K))
T: temperature of liquid nitrogen (77 K)
Cads the adsorption capacity is the amount of adsorbed molecules by unit weight of the substrate for a given temperature. The volumetric adsorptive capacity is given in Equation 4 [15]:
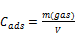 (4)
(4)
m: mass of the adsorbate (g)
V: Volume of the adsorbed monolayer (cm3)
3. Results and Discussion
3.1. Characterization of SWCNT by Nitrogen Adsorption
The acid treatment technique was used to purify the sample. The study of the adsorption of nitrogen (N2) is made in order to check whether our sample treated with nitric acid (HNO3) has indeed been purified.
After purification the sample surface area increased from 231.94 m2/g to 452.092 m2 /g [7]. The high value of the specific surface of the raw sample indicates the presence of micropores on its surface. The value has almost doubled after purification of the nanotube. Such porosity development after chemical treatment has also been observed by C. M. Yang et al [16]. They observed, during a study of the effect of oxidation after acid treatment (HCl) on the SWCNT, an increase of the specific surface area of 524 m2/g for raw nanotube HiPco to 861 m2/g for the purified nanotube.
The isothermal curves, V = f (P / P0), of the adsorption of nitrogen onto the raw and purified SWCNT at T = 77K are shown in Figure 4. V is the amount of adsorbed gas molecules, and P / Po is the relative pressure.
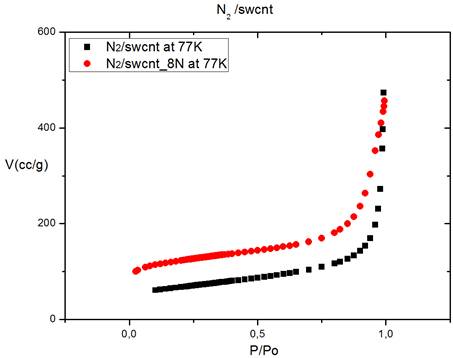
Figure 4. Adsorption of nitrogen onto raw SWCNTs and purified SWCNTs.
Those isotherms, type II, clearly show that the purified SWCNT (SWCNT_8N) adsorbs more nitrogen than raw SWCNT. To quantitatively compare the amounts adsorbed onto the raw sample and purified, a proportion of increase (X) is calculated (Equation 4).
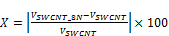 (5)
(5)
For a relative pressure P / Po of 0.75; we have: 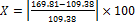
X = 55.25%. The volume of adsorbed molecules has increased 55.25% after sample purification. That increase can be attributed to the formation of new small pores or the growth of the existing pores. Those results confirm that our sample has actually been purified.
3.2. Comparative Studies of Krypton and Argon Adsorption of the SWCNT
To better understand the adsorption mechanism onto SWCNTs, adsorption of krypton and argon was performed on AP-SWCNT, both raw and purified, at the temperature of liquid nitrogen (77 K) and are showed in Figures 5 a and 5 b.
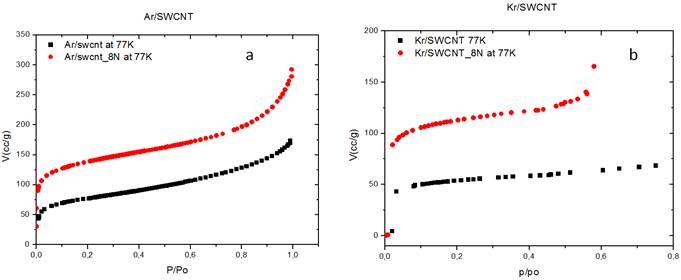
Figure 5. a) Ar adsorption onto raw SWCNTs (square) and purified SWCNTs (full circle); b) Kr adsorption onto raw SWCNTs (square) and purified SWCNTs (full circle).
Consistent with the results obtained with nitrogen, Figures 5 a and b show an increase in the quantity of adsorbed molecules (Ar and Kr) after sample purification.
To compare the results, the isothermal curves of adsorption for these two gases were overlaid (Figure 6 a and 6 b).
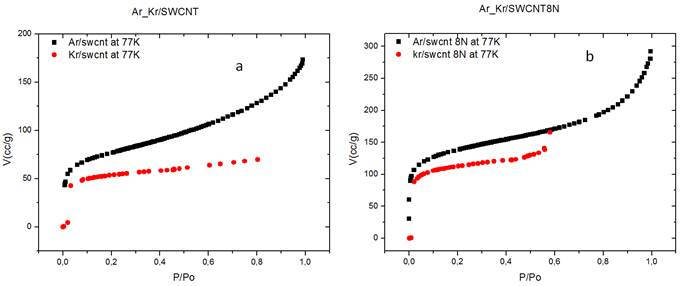
Figure 6. a) Adsorption of Ar (square) and Kr (full circle) onto raw SWCNTs. b) Adsorption of Ar (square) and Kr (full circle) onto purified SWCNTs.
In both cases, the argon molecules are better adsorbed (170.028 cc / g) by the substrate than the krypton (165.359 cc / g) for a relative pressure P / P0 of 0.6. The calculated proportion increase, X is 3%. The volume of argon molecules adsorbed is slightly greater than that of adsorbed krypton. That difference could be related to the size of the gas molecules and the occupation of the adsorption sites.
Indeed, Ar molecules have a smaller recovery cross-section (σ = 0.138 nm2) and a smaller molecular diameter (d = 0.270 nm) than those of Kr molecules (σ = 0.147 nm2; d = 0.406 nm) (Table 1) and as the size of the space offered by the interstitial channels (IC) varies; Kr molecules would therefore only have access to the broadest IC while Ar molecules would diffuse quickly in even the smallest IC. That result is in agreement with results of Moulay Rashid Babaa in his thesis [17].
Table 1. Geometrical parameters of absorbates.
| Adsorbates | Molecular diameter d (nm) | Dimension section σ (nm2) |
| Ar | 0.295 | 0.138 |
| Kr | 0.406 | 0.147 |
3.3. Adsorption Energy
The adsorption energy is the energy of substrate - adsorbate interaction added to the energy of adsorbate-adsorbate interaction. For physical adsorption (the present case) the adsorbate-adsorbate interactions are neglected; the adsorption energy can be summed up in the energy of the substrate-substrate interaction and is determined from Equation 3. The results reported in Table 2 show that the interaction energy between the substrate and Ar molecules is greater than that of the interaction energy between the substrate and the molecules of Kr [Eads (Ar) > Eads (Kr)]. Therefore it may be predicted that the adsorption energy decreases when the size of the molecules increase.
Table 2. Adsorption energies of absorbates onto raw SWCNTs and purified SWCNTs (SWCNT_8N).
| Adsorbates | d (nm) | Eads/swcnt (kJ/mol) | Eads/swcnt_8N (kJ/mol) |
| Ar | 0.295 | 4.6 | 6.1 |
| Kr | 0.406 | 3.8 | 4.0 |
Another phenomenon that could be the cause of those results is the occupation of adsorption sites. The outer surface of the substrate and the grooves are least energetic, while ICs are the most energetic micropores [18], and we can conclude that the Kr molecules are adsorbed to the outer surface of the sample while Ar molecules can be welcomed by all of the adsorption tube sites. Those results confirm results obtained by plotting isotherms in Figure 6. Similar effects have been reported by M. Seifi et al; they showed that the adsorption is best done in the most energetic site [19].
3.4. Adsorption Capacity
The adsorption capacity is the amount of adsorbed molecules by unit weight of substrate for a given temperature. Table 3 below shows the adsorption capacity of argon, krypton and nitrogen on the SWCNT.
Table 3. Adsorption capacity of Kr and Ar onto raw SWCNTs and purified SWCNT_8N.
| Adsorbate | micropores Volume (cm3/g) | Cads/swcnt (g/cm3) | Cads/swcnt_8N (g/cm3) |
| Ar | 0.011285 | 2.3 | 13.99 |
| Kr | 0.236773 | 1.35 | 1.4 |
Note that the krypton adsorption capacity of the SWCNTs is lower than that of argon on the SWCNTs. The adsorption capacity decreases when the size of the adsorbed molecules increases. Those results are in agreement with results obtained with the adsorption energy and the isotherms of adsorption.
By comparing the adsorption capacity and the volumes of the micropores, it is observed, against all expectations that the adsorption capacity of purified SWCNTs decreases when the micropore volume increases. Although in the case of the adsorption of krypton, the micropore volume of the substrate is greater than that of the substrate in the case of the adsorption of argon, and the Ar adsorption capacity is significantly higher than that of Kr. Those results suggest that krypton does not adsorb in the micropores.
4. Conclusion
This paper studied the adsorption of argon and krypton on SWCNTs at liquid nitrogen temperature (77 K). In order to determine the influence of the size of molecules on the adsorption properties and which adsorption sites could host molecules, the energies and adsorption capacities of those gases on the SWCNTs were calculated. The results obtained show that the molecules of Ar with molecular diameter of d = 0.295 nm are better adsorbed than those of Kr (d = 0.406 nm). Furthermore, all SWCNT adsorption sites could accommodate gas molecules except the larger ones will not have access to the narrower IC. This research found that the energy and the adsorption capacity increased when the size of the molecules decreases. It would be interesting to consider the implications of these results for the adsorption of hydrogen. H2 has a molecular diameter of 0.290 nm similar to that of argon. So if the mechanism defined in this study is the prevailing mechanism for the adsorption of hydrogen, H2 molecules would occupy all the adsorption sites of the SWCNT.
However, more research in this area is needed to provide a clearer understanding of adsorption phenomena on SWCNTs.
Acknowledgments
The authors are grateful for financial support from INSA-JRD TATA Fellowship D. O. /CCSTDS/1293/2008 and twas Research Grants 08-115/PHYS/AF/AC. We thank professor Aldo. D. Migone and Dr. John S. Graham for providing us help.
References
- IIJIMA S., Helical microtubules of graphitic carbon, Nature, 1991, P 56-58.
- N. Hamada, S. Shin-ichi, O. Atsushi, "New one-dimensional conductors: Graphitic microtubules", Phys. Rev. Lett., 68 (10), (1992), 1579–1581.
- E. T. Thostenson, Z. Ren, T. W. Chou, Composites Science and Technology, (2001), 61, 1899–1912
- M. Paillet, V. Jourdain, P. Poncharal, J.-L. Sauvajol, A. Zahab, J. C. Meyer, S. Roth, N. Cordente, C. Amiens, B. Chaudret Versatile, "Synthesis of individual single walled carbon nanotubes from nickel nanoparticles for the study of their physical properties", J. Phys. Chem. 108, (2004).
- Tae-jin P., Sarbajit B., Tirandai H., Stanislaus W. "Purification strategies and purity visualization techniques for single-walled carbon nanotubes" J. Mater. Chem. (2006), 141-154.
- Virginia Gomez, Silvia Irusta, Olawale B. Lawal, Wade Adams, Robert H. Hauge, Charles W. Dunnill, Andrew R. Barron, RSC Adv., (2016), 6, 11895-11902.
- A. Diama, V. Manu, M. Grafoute, H. C. Bajaj; Journal of Materials and applications, (2016), 2 (3), 25-29.
- A. Yaya, C. P. Ewels, Ph. Wagner, I. Suarez-Martinez, A. Gebramariam Tekley and L. Rosgaard Jensen, Eur. Phys. J. Appl. Phys., (2011), 54, 10401.
- K. A. G. Amankwah, J. S. Noh, J. A. Schwarz, "Hydrogen storage on superactivated carbon at refrigeration temperatures", International journal of hydrogen energy, (1989), 437-447.
- R. Q. Long, R. T. Yang, "Carbon nanotubes as a superior sorbent for nitrogen oxides", Ind. Eng., Chem. Res. (2001), 4288-4291.
- A. C. Dillon, K. M. Jones, T. A. Bekkedahl, C. H. Kiang, D. S. Bethune, M. J. Heben, "Storage of hydrogen in single-walled carbon nanotubes", Nature, (1997), 377-379.
- Srinivas Gadipelli, Zheng Xiao Guo, Progress in Materials Science, Volume 69, (2015), 1–60
- M. Muris, N. D. Pavlovsky, M. Bienfait, P. Zepperfeld, "Where are the molecules adsorbed on single walled nanotubes", Surface Science volume 992,(2001), 67-74.
- Svetlana Yu Tsareva, Edward McRae, Fabrice Valsaque, Xavier Devaux; Adsorption, (2015), Volume 21, Issue 3, 217–227.
- M. Repka, P. Lamp, "Physisorption of Hydrogen on Microporous Carbon and Carboon Nanotubes", J. Phys. Chem. B. (1998), 102, 10894-10898.
- C. M. Yang, K. Kaneko, M. Yudasaka, S. Iijima, "Effect of purification on pore structure of HiPco single walled carbon nanotube aggregates", Nano letters. 2, (2002), 385-388.
- M. R. Babaa, "Contribution à l’étude de l’adsorption physique de gaz sur les nanotubes de carbone mono- et multiparois" Thèse, (2004), 99-107.
- A. Thomy, X. Duval, J. Regnier, "Two dimensional phase transitions as displayed by adsorption isotherms on graphite and other lamellar solids", Surf. Sci. (1981), 1-38.
- M. Seifi, D. K. Ross, D. J. Riley, I. Morrison, "The dependence of the Hydrogen sorption capacity of single-walled carbon nanotubes on the concentration of catalyst", Department of Physics, University of Guilan, Rasht, 41335-1914.

 (A. Diama)
(A. Diama)