| 1. | ||
| 2. | ||
| 3. | ||
| 4. | ||
| 5. | ||
Prevalence of Urinary Schistosomiasis Among Pupils in Endemic Communities of Rivers State, Nigeria
Goodhead D. A., Dirisu C. G.*
Department of Biology Education, School of Science, Federal College of Education (Technical) Omoku, Rivers State, Nigeria
Email address
 (Dirisu C. G.)
(Dirisu C. G.) Citation
Goodhead D. A., Dirisu C. G. Prevalence of Urinary Schistosomiasis Among Pupils in Endemic Communities of Rivers State, Nigeria. American Journal of Microbiology and Biotechnology. Vol. 3, No. 2, 2016, pp. 7-12.
Abstract
A survey of urinary Schistosomiasis infection was conducted in Omokwa, Omalem and Odaga Communities of Abua/Odual Local Government Area of Rivers State to ascertain the current status of urinary Schistosomiasis. 130 urine samples were collected from individuals between the age brackets of 5-16 of both sexes. Using sedimentation techniques, 30(23.1%) were infected out of the total of 130 pupils examined, 17(22.4%) were males and 13(24.1%) were females. The highest prevalence rate of the infection was recorded among Omokwa Community 4(29.8%), while Odaga had the lowest prevalence rate of 9(22%) followed by Omalem 7(16.7%). The age group of 13-16years had the highest prevalence of 36.7%; 9-12years had 19.1% while the lowest prevalence was recorded among the age group 5-8years 18.9%. X2 statistic indicated that there is significant difference of infection within the age and sex groups of the pupils (p>0.05). Effective and sustainable infection with S. haematobium control and management strategies for the study areas is recommended.
Keywords
Cercariae, Schistosoma, Urinary Schistosomiasis, Schistosomiasis
1. Introduction
Schistosomiasis or bilharzia is a tropical parasitic disease caused by blood-dwelling fluke worms of the genus Schistosoma. It is a very serious environmental health problem in many tropical countries. Schistosomiasis is a clinical term applied to infection with one of a series of related trematode parasites that are endemic to 76 tropical and sub-tropical countries [1]. According to [2], about 200-300 million people may be suffering from the disease worldwide. Schistosomiasis is very focal and determined by the presence of competent snail vector, inadequate sanitation and infected human [3], [4]. The disease is endemic in Nigeria [5], [6].
In developing countries, the disease affects many people particularly school-aged children between 10 and 14, who are mostly at risk as they tend to spend time bathing and swimming in infected water containing infectious cercariae. Adult workers in rural areas employed either in agriculture or the fresh water fishing section are also infected. Udonsi, [7] also stated that water contact activities and traditional agricultural practices are also the factors in the distribution of the disease and its snail hosts in the developing countries.
In terms of impact, this disease is second only to malaria as the most devastating parasitic disease and is also considered one of the neglected tropical diseases (NTDS) in the world [6]. The parasites that cause Schistosomiasis live in certain types of freshwater snails. The infective form of the parasite, known as cercariae, emerge from the snail, one can become infected when the skin comes in contact with contaminated fresh water. Most human infections are caused by S. haematobium, S. mansoni and S. Japonicum. 200 million people are said to be infected worldwide, leading to the loss of 1.53 million disability-adjusted life years (DALYS) [8].
Schistosomiasis is characterized by focal epidemiology and over dispersed population distribution, with higher infection rates in children than in adults [9]. [9] illustrated that complex immune mechanisms leads to the slow acquisition of immune resistance, though innate factors also plays a part. Acute Schistosomiasis, a feverish syndrome, is mostly seen in travelers after primary infection with Schistosomiasis. Chronic schistosomal disease affects mainly individual with long standing infections in poor rural areas. That is to say that chronic complication are generally seen in those with a high parasite load, which usually occurs in individual who live in endemic areas and have recurrent exposure to the infection
Urinary schistosomiasis is caused by Schistosoma haematobium and is a major cause of considerable morbidity, mortality and debility in humans [10], [13]. In S. haematobium infection, the adult schistosomes preferentially localize to the veins of the kidneys, urethras and bladder. During infection, the parasites deposits terminal spine eggs which clog the venous plexus, impeding blood flow, this burst the veins, allowing blood and eggs to enter the urinary bladder, resulting in the characteristic symptom of blood in urine [11]. Factors such as water contact occupations, rapid urbanization, poor sanitation, population increase, account for the endemicity of urinary schistosomiasis in Nigeria [12]. No single method of control strategies of schistosomiasis, regardless of location, has been shown to work because of the large number of environmental variables involved in its transmission. For these reasons, [2], [10] mapped out four approaches (integrated), which combined population based chemotherapy-employing praziquantel, public health education, sanitation and control of snail vector. In view of the public health concern and economic importance of Schistosomiasis, this study was carried out to determine the prevalence of S. haematobium in Omokwa, Omalem and Odaga Communities, determine specific age and sex prevalence of infection in the study areas, in order to provide data for surveillance and control programmes.
2. Materials and Methods
Study Areas
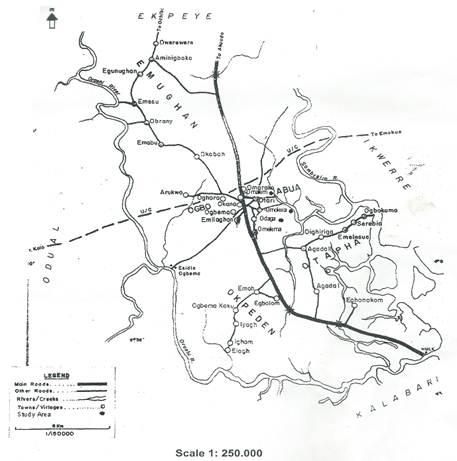
Fig. 1. Map of Abua/Odual L. G. A of Rivers State showing the study areas.
The research was carried out in Abua/Odual Local Government Area of Rivers State; an ethnic nationality of over 300,000 people with an estimated area of 300 square Kilometers. Specifically the study was done in three communities which are Omokwa, Omalem and Odaga Communities in the clans of Ogbo-Abuan located geographically between Latitudes 4.5 and 6.5 Degrees North of the Equator and longitudes 6.0 and 7.0 Degrees East of Greenwich Meridian (Fig 1). Vegetation type is tropical rain forest. Rainy season is experienced between March and October, which peaks in September while dry season is experienced between November and February. The people of Abua are predominantly farmers and fishermen. They produce plantain and banana, pineapples in commercial quantities. Aquatic resources such as freshwater fishes and snails are produced.
Sample
The sample used for the study was urine specimen collected from one hundred and thirty (130) pupils in Omokwa, Omalem and Odaga community schools. Of these 130 samples collected 54 were males and 76 were females from aged 5-16 years of both gender (male and female).
Collection of sample
During sampling, permission was sought from the school Headmasters. Also, relevance of the study especially the public health significance was made known to both the pupils and Headmasters. Thereafter, clean dry Screw-Capped urine container was given to each pupil to provide a terminal urine specimen between the hour of 1-2; which they did and later returned to the researcher to preserve with 5ml of 10% formalin to prevent the eggs of Schistosomes from hatching or degenerating. The collected urine samples were transported to the parasitological Research Laboratory of the University of Port-Harcourt for immediate parasitological examination of the urine.
Examination of Urine
Using Sedimentation technique, 10ml of the urine sample was centrifuged at 1,500rpm for 5 minutes. The urine was poured into a graduated centrifuge tube and centrifuged. The supernatant was discarded of leaving the sediment. The sediments were mixed thoroughly and pipetted out into a clean grease free slide and covered with cover slips.
The slide were viewed using x10 objectives and then x40 objective of a light Microscope to identify S. haematobium eggs characterized with a terminal spine [13], [4] (see Figure A1 in appendix).
Statistical Analysis
The data was entered and analyzed using percentage and chi-square statistical analysis to analyze the result obtained and to test for the significance in difference in the prevalence of infection within the age groups and sexes among the pupils in the three communities.
3. Result and Discussion
PREVALENCE OF S. haematobium AMONG PUPILS
Table 1 depicts the status of urinary Schistosomiasis in Omokwa, Omalem and Odaga Communities. Of the combined total of 130 pupils examined, 54 were males and 76 were females. The overall status of S. haematobium infection was 30 (23.1%), out of the 30 infected, 14(29.8%) were from Omokwa, 7(16.7%) from Omalem and 9(22%) were from Odaga Community.
Table 1. Prevalence of S. haematobium in the study area.
| Male | Female | Total | ||||
| Sampling site | No. Examined | No. (%) Infected | No. Examined | No. (%) Infected | No. Examined | No. (%) infected |
| Omokwa | 20 | 6(30%) | 27 | 8(29.6%) | 47 | 14(29.8) |
| Omalem | 13 | 3(23.1%) | 29 | 4(13.8%) | 42 | 7(16.8) |
| Odaga | 21 | 4(19.1%) | 20 | 5(25%) | 41 | (9(22%) |
| Total | 54 | 13(24.1%) | 76 | 17(22.4%) | 130 | 30(23.1%) |
INTENSITY OF INFECTION WITH S. haematobium
Table 2. Intensity of Schistosoma haematobium infection
| Male | Female | |||||
| Communities | No. (%) with light infection (<50) | No. (%) with heavy infection ( 50 >100) | Total infected | No. (%) with light infection (<50) | No. (%) with heavy infection ( 50 >100) | Total infected |
| Omokwa | 17(85%) | 3(15%) | 20 | 24(88.9%) | 3(11.1%) | 27 |
| Omalem | 13(100%) | 0(0%) | 13 | 29(100%) | 0(0%) | 29 |
| Odaga | 21(100%) | 0(0%) | 21 | 20(100%) | 0(0%) | 20 |
| Total | 51(94.4%) | 3(5.6%) | 54 | 73(96.1%) | 3(3.9%) | 76 |
Table 2 depicts the intensity of infection as regards to the worm burden in the study areas. Two communities – Omalem and Odaga recorded low worm load, indicating light infection of S. haematobium whereas, Omokwa community recorded heavy worm load indicating high intensity of infection.
AGE- SPECIFIC PREVALENCE OF INFECTION IN THE STUDY AREAS
Table 3. Age prevalence of S. haematobium in the study areas.
| No. examined | No (%) Infected | |||||||
| Age Group | Omokwa | Omalem | Odaga | Group Total | Omokwa | Omalem | Odaga | Group Total |
| 5-8 | 21 | 18 | 14 | 53 | 6(28.6%) | 2(11.1%) | 2(14.3%) | 10(19.1) |
| 9-12 | 17 | 13 | 17 | 47 | 4(23.5%) | 1(7.7%) | 4(23.5%) | 9(19.1%) |
| 13-16 | 9 | 11 | 10 | 30 | 4(44.4%) | 4(36.4%) | 3(30%) | 11(36.7%) |
| Total | 47 | 42 | 41 | 130 | 14(29.8%) | 7(16.7%) | 9(30.0%) | 30(23.1%) |
Table 4. Sex prevalence of S. haematobium among pupils in Omokwa, Omalem and Odaga Communities.
| No. examined | No. infected | |||||||
| Sex Groups | Omokwa | Omalem | Odaga | Group Total | Omokwa | Omalem | Odaga | Group Total |
| Male | 20 | 13 | 21 | 54 | 6(30%) | 3(23.1%) | 4(19.1%) | 13(24.1) |
| Female | 27 | 29 | 20 | 76 | 8(29.6%) | 4(13.8%) | 5(25%) | 17(22.4%) |
| Total | 47 | 42 | 41 | 130 | 14(29.8%) | 7(16.7%) | 9(30.0%) | 30(23.1%) |
The age prevalence of infection in the study areas is showed in Table 3. Pupils within 13-16years age bracket had the highest prevalence of 36.7%, followed by the pupils in 9-12years age bracket group with prevalence of 19.1% while those within 5-8years age group had the lowest prevalence of 18.9%.(![]() Statistic indicated a significant difference of infection between the age group of pupils ((
Statistic indicated a significant difference of infection between the age group of pupils ((![]() Calculated = 14.58, > 5.991, P< 0.05).
Calculated = 14.58, > 5.991, P< 0.05).
SEX- SPECIFIC PREVALENCE OF INFECTION IN THE STUDY AREAS
Table 4 shows sex prevalence of infection among the pupils in the study areas. Of the 54 males and 76 females examined, 30 were infected, out of which 13(24.1%) males and 17(22.4%) females were infected. This indicates that female had higher prevalence of the infection with S. haematobium than males. There was significant difference in the prevalence of infection among the different sex groups. (![]() Calculated = 15.55 >
Calculated = 15.55 >![]() tabulated 3.841 under P < 0.05 df).
tabulated 3.841 under P < 0.05 df).
4. Discussion
The results of this survey which indicated S. haematobium infection overall prevalence rate of 23.1% in Omokwa, Omalem and Odaga communities suggest that Abual/Odual Local Government Area of Rivers State falls within the WHO classification as endemic. The present study supports a number of previous reports which have consistently shown that S. haematobium infection endemicity in Rivers State and Nigeria as a whole is in the increase, particularly in the rural areas with school aged child at greatest risk [14], [15]. The prevalence rate reported in this study is lower than 62% in South –Western Nigeria [16], 54.6% in Ogun state [17], 41.6% in Danjarima, Kano State [18], 41.5% in Buruku and Kastina-Ala, Benue state [19] and 37.9% in Sankwala, Cross River state [20]. In contrast, the PR was higher than 18.7% in Niger-Benue Basin, Kogi state [21] and 10.4% in Ohaji-Egbema [22]. Beyond this country, PR of 60%, 6.9%, 32.1%, 10.4% and 50.8%, have been reported for Zimbabwe [23], Malawi [24], Kumba, Cameroun [25], Blantyre, Malawi [26] and South-West Cameroun [27] respectively. Poverty, ignorance, poor living conditions, inadequate sanitation and water supplies as well as deplorable personal and environmental hygiene characteristics of many rural communities in Nigeria are identified as importance factors contributing to the transmission of schistosomiasis [28]. The major factors that may have been responsible for the endemicity of urinary schistosomiasis in the study areas could be lack of management of the infection, lack of basic amenities, inadequate and indiscriminate disposal of human sewage and high water contact activity with snail- infested ponds, streams and lakes. This observation supports the recommendation by the WHO expert community [29], on the reduction of schistosomiasis prevalence by the use of operational components such as adequate water supply, sanitation and environmental management.
The study showed that females were generally more infected than males in the three communities. This is similar to the observation made by [30] but contrast to the report in Ohaukwu and Onicha local government areas by Nmorsi et al [31] and Benue state [12] which showed that males are more infected than females, presumably due to higher water contact activities.
5. Conclusion
In this study, it was observed that Omalem and Odaga communities recorded low worm burden of S. haematobium indicating light intensity of infection whereas Omokwa recorded heavy worm load indicating heavy intensity of infection. This indicated that the distribution of Schistosomiasis in endemic communities fits a negative binomial curve with most infected persons harbouring low worm burdens and only a small proportion having heavy infections. However, the aggregation of worm burdens in a small proportion of infected individuals may have multiple explanations including genetic susceptibility [32].
Appendix
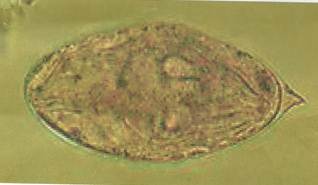
Figure A1. Egg of Schistosoma haematobium.
References