Study of Biofilm Production and Antimicrobial Resistance Pattern of Klebsiella Pneumoniae Isolated from Urinary Catheter at the University Hospital of Tlemcen
Samia Bellifa*, Hafida Hassaine, Ibtissem Kara Terki, Wafae Didi, Imene M’hamedi, Meriem Lachachi, Ibrahim Benamar,
Touhami Morghad, Sarah Gaouar
Laboratory of Food, Biomedical and Environmental Microbiology (LAMAABE), University of Tlemcen, Tlemcen, Algeria
Email address

(S. Bellifa)
*Corresponding author
Citation
Samia Bellifa, Hafida Hassaine, Ibtissem Kara Terki, Wafae Didi, Imene M’hamedi, Meriem Lachachi, Ibrahim Benamar, Touhami Morghad, Sarah Gaouar. Study of Biofilm Production and Antimicrobial Resistance Pattern of Klebsiella Pneumoniae Isolated from Urinary Catheter at the University Hospital of Tlemcen. American Journal of Microbiology and Biotechnology. Vol. 3, No. 2, 2016, pp. 13-17.
Abstract
Klebsiella pneumoniae is an important gram-negative opportunistic pathogen causing primarily urinary tract infections, respiratory infections, and bacteraemia. The ability of bacteria to form biofilms on medical devices, e.g. catheters, has a major role in development of many nosocomial infections. There are various methods to detect biofilm production like Tissue Culture Plate (TCP), Tube method (TM), Congo Red Agar method (CRA), bioluminescent assay, piezoelectric sensors, and fluorescent microscopic examination. In the present studywe screened 100 strains of K. pneumoniae isolated from urinary catheter at the university hospital of Tlemcen (Algeria) by three methods for the detection of biofilms (TCP, TM, CRA). Antibiotic susceptibility test of biofilm producing bacteria was performed by using the Kirby-Bauer disc diffusion technique according to CLSI guidelines. The TCP method was considered to be superior to TM and CRA. From the total of 100 clinical isolates, TCP method detected 69% as high, 21% moderate and 10% as weak or non-biofilm producers. We have observed higher antibiotic resistance in biofilm producing bacteria than non-biofilm producers. This study demonstrates a high propensity among the clinical isolates of K. pneumoniae to form biofilm and a significant association of biofilm with multiple drug resistance.
Keywords
Biofilm, Klebsiella pneumoniae, TCP, TM, RCA
1. Introduction
Biofilm is a community of microorganisms embedded in a self produced polymeric matrix comprising of polysaccharides, proteins, glycopeptides, nucleic acids and lipids [1,2]. Currently, it is estimated that over 60% of bacterial infections and up to 80% of chronic infections involve microbial growth in biofilms [3]. Biofilm formation is a complex process that can be subdivided into relatively distinct phases: primary attachment of cell, cell-tocell adhesion and proliferation, biofilm maturation, and finally detachment of planktonic cell from the biofilm. It has been demonstrated in vitro that bacteria growing within biofilms are more resistant to antibiotic treatment than bacteria growing planktonically, the bacteria here are 100 to 1,000 times more tolerant to antimicrobials than corresponding planktonic cells [4].
Klebsiella pneumoniae is an opportunistic pathogen associated with both community-acquired and nosocomial infections, including pneumonia, urinary tract infections, septicemia and wound infections, with the increasingly multidrug-resistant (MDR) K. pneumoniae being a major public health concern [5]. Virulence factors that are associated with Klebsiella pathogenesis include the production of an antiphagocytic capsule as well as lipopolysaccharide (LPS) that contributes to serum resistance, siderophores to capture host iron, and type 1 and type 3 fimbriae that allow the bacteria to bind to host structures [6]. Recent studies suggest that biofilm formation may also be an important virulence factor for K. pneumoniae [7].
There are different methods to detect the production of a biofilm. These include mainly the tissue culture plate method (TCP)[8], the tube method (TM) [9], the Congo Red Agar method (CRA) [10] and the bioluminescent assay [11].
In the present study, we examined 100 strains of Klebsiella pneumoniae isolated from urinary catheters during various services at the University Hospital, using three different methods to assess their ability to form a biofilm in vitro.
2. Materials and Methods
2.1. Strains of K. pneumoniae Used
From May 2013 to May 2015, 140 patients were included in this study, where 150 urinary catheters were collected from four services at the university hospital of Tlemcen (urology, intensive care unit ICU, surgery, and neurosurgery). Only implanted urinary catheters for 48 hours or more were taken. Medical devices were carefully removed under aseptic conditions, placed individually in sterile glass tubes and transported immediately to the laboratory for analysis.
The distal end of each catheter was cut (4cm), placed in one mL of sterile saline, sonicated and vortexed for 1 minute. A volume of 100µL was inoculated on Mac Conkey medium used for the isolation of gram-negative bacteria. The identification of strains is controlled, after checking their purity, by the study of macroscopic and microscopic characters (form colonies, mobility, Gram staining) and biochemical (oxidase test, catalase test, API 20E (Biomerieux ®).
2.2. Anti-microbial Susceptibility Testing
K. pneumoniae isolates were selected to determine their susceptibility patterns, by the disc diffusion method of Kirby- Bauer as described by Clinical Laboratory Standards Institute (CLSI) [12]. The antimicrobial agents used were: amoxicillin®, Amoxicillin/clavulanic® acid. Cefotaxime®, ceftazidime®, nalidixic acid®, tetracycline®, gentamicin®, tobramycin, ciprofloxacin®, and ofloxacin®.
2.3. Tissue Culture Plate Method
Biofilm formation K. pneumoniae of cell on polystyrene was quantified by using the microtiter plate assay [13,14]. Isolates were cultivated overnight in 96-well flat-bottomed tissue culture plates at 37°C with trypticase soy broth (bio- Mérieux, France). After incubation, the cultures were gently removed, the wells were washed three times with phosphate buffered saline (pH 7.3), and air dried and stained with 0.4% crystal violet solution for 10 min. The plate was washed, the adherent cells were resuspended in ethanol (70%), and finally the absorbance at 490 nm was determined by an ELISA plate reader (Expert, Plus, Asys). The interpretation of biofilm production was done according to the criteria of [15]. Each isolate was tested in triplicate.
2.4. Tube Method
Described by Christensen et al. (1982) [9] this is a qualitative method for biofilm detection. A loopful of test organisms was inoculated in 10 mL of trypticase soy broth in test tubes. The tubes were incubated at 37°C for 24 h. After incubation, tubes were decanted and washed with phosphate buffer saline and dried. Tubes were then stained with crystal violet (0.4%). Excess stain was washed with deionized water. Tubes were dried in inverted position. The scoring for tube method was done according to the results of the control strains. Biofilm formation was considered positive when a visible film lined the wall and the bottom of the tube. The amount of biofilm formed was scored as 1-weak/none, 2-moderate and 3-high/strong. The experiment was performed in triplicate and repeated three times.
2.5. Congo Red Agar Method
Freeman et al (1989) [10] have described a simple qualitative method to detect biofilm production by using Congo Red Agar (CRA) medium. CRA medium was prepared with brain heart infusion broth 37 g/L, sucrose 50 g/L, agar No. 1 10 g/L and Congo Red indicator (Oxoid, UK) 8 g/L. First Congo red stain was prepared as a concentrated aqueous solution and autoclaved (121°C for 15 minutes) separately from the other medium constituents. Then it was added to the autoclaved brain heart infusion agar with sucrose at 55°C. CRA plates were inoculated with test organisms and incubated at 37°C for 24 h. Black colonies with a dry crystalline consistency indicated biofilm production [16]. The experiment was performed in triplicate and repeated three times.
3. Results
3.1. Characterization of Isolates
A number of 100 isolates of K. pneumoniae were collected from 150 urinary catheters. The API 20E analysis allowed the characterization of four biotypes: 5215773, 5205773 (urea -), 5214773 (VP-), and 52,155,573 (inositol -).
K. pneumoniae isolates were maximally resistant (100%) to amoxicillin and Amoxicillin/clavulanic acid. A moderately high resistance of 66.66%, 58.33% were shown cefotaxime and ceftazidime, respectively. Regarding the aminoglycosides, a 50% resistance rate is observed for nalidixic acid. A fairly stronger resistance is noted to tetracycline, gentamicin and tobramycin, that is 82%, 83.33% and 66%, respectively. An excellent activity of fluoroquinolones: ciprofloxacin and ofloxacin is observed, with resistance rates equal to 10% and 6.6%.
3.2. Screening of K. pneumoniae Isolates for Detection of Biofilm Formation
The 100 clinical K. pneumoniae strains isolated in our study were tested for their ability to form biofilms by three different techniques: Tissue Culture Plate method (TCP), Tube Method (TM) method of Congo Red Agar (CRA). Comparison of biofilm production by clinical isolates of S. aureus by three conventional methods is given in Table 1.
The microtitre plate test correctly identify both the positive and the negative reference bacterial strains. Sixty nine out of 100 strains (69%) were found to be biofilm producers with an OD within the range [0.56 - 2.5], 21 (21%) strains are moderate with an OD in the range [0.28 - 0.56], followed by 10 (10%) low producing strains with a OD ranging from 0.15 to 0.28.
The detection of biofilm formation using TM has shown that 50 strains (50%) were biofilm forming, 28 (28%) moderate, and 22 strains (22%) were non-biofilm-forming (Figure 1).

Figure 1. Biofilm formation on glass surfaces under static growth conditions.
Tube C: Control, Tube 1: non adherent, tube 2: weakly adherent, tube 3: strongly adherent.
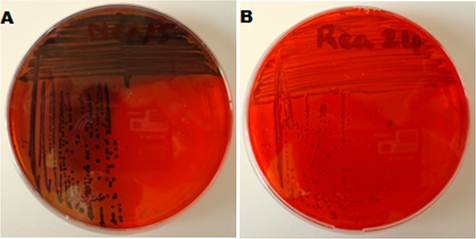
By Congo red agar method a total of 40% of the strains (n = 40) were producers of black colonies with absence of dry crystalline colonial morphology and 30 strains were classified as non-producers (smooth red colonies) (Figure 2).
4. Discussion
Biofilm formation on medical instruments is becoming a problem of increasing health and economic concern [1,2]. Indwelling medical devices such as sutures, catheters, heart valves, vascular grafts, orthopedic implants, and intrauterine devices, are prone to biofilm formation, leading to a major risk of infection and patient morbidity. The presence of a protein film on medical implants in direct contact with a fluid promotes adhesion and biofilm formation by K. pneumoniae, which results in resistance to the host’s immune system and antibiotics [17]. In view of the large number of infections caused by biofilm- producing bacterial, a reliable method for their diagnosis is necessary. In the present work, the ability of biofilm formation by Klebsiella pneumoniae is evaluated using three techniques: TCP, TP, and CRA.
100 strains of Klebsiella pneumoniae were isolated from 150 urinary catheters, the persistence of this species in hospitals is partly due to its ability to adhere and multiply on inanimate surfaces. The resistance profile in this study revealed a remarkable resistance to most of the antibiotic agents tested, The increasing prevalence of clinical multi-drug resistant isolates of K. pneumoniae has been associated with higher morbidity and mortality rates, posing a considerable threat to public health.
According to the microplate technique, most strains were considered to be strong biofilm-forming. Oliveira and Cucha confirmed the relatively high efficiency of the microplate method [18]. The Microtitre plate test is a convenient and economical quantitative technique for the identification of critical factors and optimal culture conditions for biofilm formation. This technique is used for direct detection of polysaccharide production because spectrophotometric measurements provide quantitative information on the ability of bacterial strains to rapidly grow while adhering to the substratum [19]. However, it can be less accurate in determining their specific ability to secrete PIA because, while it is a very sensitive test, it has low specificity [15]. Revdiwala and colleagues undertook a broad examination of biofilm formation by TCP method by isolates recovered from a variety of medical support devices. One hundred isolates were recovered, including K. pneumoniae, Crystal Violet staining identified 69 biofilm formers [20].
The tube test correlates well with the TCP test for strongly biofilm producing isolates but it was difficult to discriminated between weak and biofilm negative isolates due to the variability in observed results by different observers. Furthermore, Mathur et al. (2007) [21] detected the formation of biofilms by TM in uropathogenics, according to their results, 75% of isolates are considered formative. In agreement with the previous reports, tube test cannot be recommended as general screening test to identify biofilm-producing isolate [22]. The TM method is easy to perform but reading the results may be difficult. Consequently, high variability was observed and classification in biofilm positive and negative was difficult by tube method. In CRA method, out of eight positive isolates, 40 (40%) displayed black colonies with dry crystalline morphology, and 30 (30%) displayed dry crystalline morphology. The RCA exhibited a low correlation with the TCP method. In a study [23], of 147 S. epidermidis isolates, the TM method detected the formation of biofilms in 79 (53.7%) and the CRA method detected biofilm formation in 64 (43.5%). It was shown that TM is a better technique for biofilm detection compared to CRA.
The results of the microtitre plate test were compared with those of the CRA test, and the results of the microtitre plate test indicate that it was better than the CRA test in the detection of biofilms in vitro, because of it’s a higher sensitivity in detecting the positive strains.
It is noted that the TCP technique can be considered as the most reliable to detect the ability to form biofilm in vitro compared to TM and CRA.
The present study also showed significant correlation between biofilm production and multidrug resistance. Antibiotic therapy against device associated biofilm organisms often fails without the removal of the infected implant [24]. Microbial biofilms have been associated with a variety of persistent infections which respond poorly to conventional antibiotic therapy. This also helps in the spread of antibiotic resistant traits in nosocomial pathogens by increasing mutation rates and by the exchange of genes which are responsible for antibiotic resistance.
5. Conclusion
A number of 100 isolates of K. pneumoniae were collected from 150 urinary catheters from various services at the University Hospital. K. pneumoniae is a frequently encountered hospital-acquired opportunistic pathogen that typically infects patients with indwelling medical devices. We can conclude from our study that TCP is a quantitative and reliable method to detect biofilm formation. K. pneumoniae strains isolated are very good biofilm producers which increases its pathogenicity.
The microbial biofilms may pose a public health problem for the persons who require indwelling medical devices, as the microorganisms in the biofilms are difficult to treat with antimicrobial agents. In order to limit the colonization of urinary catheters, some preventive measures should be taken into consideration, namely regular urinary catheterization, strict application of aseptic rules, hygienic measures and the right choice of biomaterials.
Acknowledgements
The authors would like to thank Dr Benabadji teacher of English at the University of Tlemcen for reviewing the article language, and we are extremely grateful to the physicians at the university hospital of Tlemcen for their help to recover the samples.
References
- Francolini I, Donelli G. Prevention and control of biofilm-based medical-device-related infections. FEMS Immunol Med Microbiol 2010; 59(3):227–238.
- Sousa C, Henriques M, Oliveira R. Mini-review: Antimicrobial central venous catheters recent advances and strategies. Biofouling 2011; 27(6):609–620.
- Mack D, Rohde H, Harris LG, Davies AP, Horstkotte MA, Knobloch JK-M. Biofilm formation in medical device-related infection. Int J Artif Organs 2006; 29:343–359.
- Olsen I. Biofilm-specific antibiotic tolerance and resistance. Eur J Clin Microbiol Infect Dis 2015; 34(5): 877-886.
- Cao X, Xu X, Zhang Z, Shen H, Chen J, Zhang K. Molecular characterization of clinical multidrug-resistant Klebsiella pneumoniae isolates. Ann Clin Microbiol Antimicrob 2014; 13(1): 1.
- Boddicker J. D, Anderson R. A, Jagnow J, Clegg S. Signature-tagged mutagenesis of Klebsiella pneumoniae to identify genes that influence biofilm formation on extracellular matrix material.Infect Immun2006; 74(8):4590-4597.
- Murphy C. N, Clegg S. Klebsiella pneumoniae and type 3 fimbriae: nosocomial infection, regulation and biofilm formation. Future Microbiol 2012; 7(8): 991-1002.
- Christensen GD, Simpson WA, Younger JA et al. Adherence of coagulase negative Staphylococci to plastic tissue cultures: a quantitative model for the adherence of Staphylococci to medical devices.J Clin Microbiol1995;22:996-1006.
- Christensen GD, Simpson WA, Bisno AL, Beachey EH. Adherence of slime producing strains ofStaphylococcus epidermidisto smooth surfaces.Infect Immun1982;37:318-26.
- Freeman J, Falkiner FR, Keane CT. New method for detecting slime production by coagulase negative staphylococci.J Clin Pathol1989;42:872-4.
- Donlan RM, Murga R, Bell M et al. Protocol for detection of biofilms on needleless connectors attached to central venous catheters. J Clin Microbiol2001;39:750-3.
- Clinical Laboratory Standards Institute: Performance standards for antimicrobial susceptibility testing; Twenty First informational supplement (M 100-S21). Wayne PA: Clinical and Laboratory Standards Institute; 2011.
- Kim J, Kim C, Hacker J, Ziebuhr W, Lee BK, Cho S. Molecular characterization of regulatory genes associated with biofilm variation in a Staphylococcus aureus strain. J Microbiol Biotechnol 2008; 18:28–34.
- Fredheim EGA, Klingenberg C, Rodhe H, Frankenberger S, Gaustad P, Fllaegstad T, Sollid JE. Biofilm formation by Staphylococcus haemolyticus. J Clin Microbiol 2009; 47:1172–1180.
- Stepanovic S, Vukovi D, Hola V et al. Quantification of biofilm in microtiter plates: overview of testing conditions and practical recommendations for assessment of biofilm production by Staphylococci.APMI2007;115:891-9.
- Reid G. Biofilms in infectious disease and on medical devices.Int. J. Antimic Ag1999;11:223-226.
- Henequen C, Forestier C. Influence of capsule and extended-spectrum beta-lactamases encoding plasmids upon Klebsiella pneumoniae adhesion. Res Microbiol 2007; 158: 339-347.
- Oliveira A, Cunha M.L.R.S. Comparison of methods for the detection of biofilm production in coagulase-negative staphylococci. BMC Res 2010; 10.1186/1756-0500-3-260.
- Melo P. D. C, Ferreira L. M, Nader Filho A, Zafalon L. F, Vicente H. I. G, Souza V. D. Comparison of methods for the detection of biofilm formation by Staphylococcus aureus isolated from bovine subclinical mastitis. Braz. J. Microbiol 2013; 44(1): 119-124.
- Baqai R, Aziz M, Rasool G. Urinary tract infection in diabetic patients and biofilm formation of uropathogens.Infect Dis J Pakistan2008;7(1):7-9.
- Mathur T, Singhal S, Khan S, Upadhyay D. J, Fatma T, Rattan A. Detection of biofilm formation among the clinical isolates of staphylococci: an evaluation of three different screening methods. Indian J Med Microbiol 2006; 24(1), 25.
- Revdiwala S, Rajdev BM, Mulla S. Characterization of bacterial etiologic agents of biofilm formation in medical devices in critical care setup. Crit Care Res Pract 2012:945805.
- Wojtyczka R. D, Orlewska K, Kępa M, Idzik D, Dziedzic A, Mularz T, Wąsik T. J. Biofilm formation and antimicrobial susceptibility of Staphylococcus epidermidis strains from a hospital environment. Int J Environ Res Public Health 2014; 11(5): 4619-4633.
- Percival S. L, Suleman L, Vuotto C, Donelli G. Healthcare-associated infections, medical devices and biofilms: risk, tolerance and control. J Med Microbiol 2015; 64(4): 323-334.

 (S. Bellifa)
(S. Bellifa)