Survival and Metabolic Characteristics of Lux-Marked Escherichia Coli O157:H7 in Different Types of Milk
Rabya A. Lahmer1, *, Nawfal A. Alhelfi2, Davey L. Jones3,
A. Prysor Williams3
1Department of Food Science and Technology, Faculty of Agriculture, University of Tripoli, Tripoli, Libya
2Department of Food Science, Agriculture College, University of Basra, Basra, Iraq
3School of Environment, Natural Resources & Geography, College of Natural Sciences, Bangor University, UK
Email address

(R. A. Lahmer)
*Corresponding author
Citation
Rabya A. Lahmer, Nawfal A. Alhelfi, Davey L. Jones, A. Prysor Williams. Survival and Metabolic Characteristics of Lux-Marked Escherichia Coli O157:H7 in Different Types of Milk. American Journal of Microbiology and Biotechnology. Vol. 3, No. 3, 2016, pp. 23-28.
Abstract
Escherichia coliO157:H7 is a potentially lethal pathogen which has been responsible for several outbreaks of milk-borne illness in recent years. The objective of this study was to evaluate the survival and metabolic activity (indexed by bioluminescence) of a chromosomally lux-marked strain of E. coli O157:H7 in raw, pasteurized and microfiltered pasteurized milk at 4 and 20°C for up to 14 d. Results showed that the population of E. coli O157:H7 and its metabolic activity decreased in all samples during storage at 4°C, with no significant differences in numbers observed between the different milk types; but metabolic activity was significantly higher (P<0·05) in the microfiltered pasteurized milk than that in raw milk. At 20°C, E. coli O157:H7 counts and cell activity peaked at day 2, and then declined progressively. At 20°C, survival and metabolic activity were significantly lower in raw milk compared with pasteurized milk. We conclude that storage temperature is more important in regulating the survival of E. coli O157:H7 in contaminated milk than its origin/pre–treatment conditions.
Keywords
1. Introduction
Due to the high nutrient content of milk, it is an optimal medium for the growth of several microorganisms [1]. Consumption of raw milk, if not heat–treated or pasteurized, can be particularly problematic and is responsible for many disease outbreaks worldwide. Outbreaks are also associated with improperly pasteurized milk, and dairy products made from unpasteurized milk [2, 3].
Escherichia coli O157:H7 was first identified as a human pathogen in 1982 when outbreaks of bloody diarrhea and severe abdominal cramps occurred in the USA [4]. The majority of affected individuals are children and the elderly, who can develop complications including haemorrhagic diarrhoea, haemolytic uremic syndrome, and thrombotic thrombocytopenic purpura [5]. Though only a small percentage of raw milk samples have been found to be E. coli O157:H7 positive [6], contamination with this pathogen has resulted in several milk–borne outbreaks of gastroenteritis [7]. Since E. coli O157 is an ordinary inhabitant of the bovine intestinal tract, the route of contamination with E. coli O157:H7 is through faecal contact with feedstuffs, or during milking without strict hygiene practices [8].
To date, the milk industry has successfully tackled issues of milk safety through various intervention strategies. Pasteurization has proved to be an effective measure in ensuring the safety of milk and dairy products. While unpasteurized raw milk can pose a public health concern, post–pasteurization contamination with E. coli O157 should also be noted. Faulty on–farm pasteurizers have also resulted in an outbreak of E. coli O157 [9]. Incidentally, microbial growth has been shown to be greater in pasteurized samples of whey than its unpasteurized counterpart at a range of storage temperatures [10]. Therefore, effective pasteurization and avoiding post–pasteurization cross–contamination in the fridge environment are both necessary to ensure the safety of milk and milk products [11].
Although human pathogen outbreaks associated with milk are relatively rare, it is important to minimise this threat to maintain consumer confidence in dairy products and to protect the dairy industry. To date, no studies have examined the metabolic activity of E. coli O157:H7 in different types of milk during storage, an important evaluator of the pathogen's potential infectivity [12]. The present paper reported an experiment to explore the survival and metabolic activity of lux-marked E. coli O157:H7 in raw milk and different types of pasteurized milk at ambient temperature (about 20°C) and at refrigeration temperature (about 4°C) conditions. To do this, a bioluminescent (lux–marked) strain of E. coli O157:H7 (strain 3704 Tn5 luxCDABE; [13] was used. Measuring bioluminescence in relative light units (RLU) indicates the degree of cellular metabolic activity [13], and has proved to be useful in improving our understanding of the pathogen in a range of contrasting environments (e.g. [12,14,15,16]).
2. Materials and Methods
2.1. Preparation of Milk
Raw milk was collected from the tank of a dairy farm located in Bangor, North Wales. The samples were kept at 4°C in sterile ice bags during transportation. Milk was used within 3 h after arrival at the laboratory. Part of the raw milk remained unpasteurized, whilst part was heat–treated in glass containers to 63·5°C (30 min) to prepare laboratory-pasteurized milk. Fresh full–fat commercially-pasteurized and full-fat microfiltered pasteurized milk (Cravendale) were purchased from Arla Foods UK Ltd (Leeds, UK).
2.2. Screening Milk Samples for E. Coli O157:H7
Milk samples were tested for the presence of E. coli O157 before inoculation. Isolation and detection of E. coli O157:H7 involved enrichment followed by immunomagnetic separation (IMS). To start with, 5 ml of each milk samples were mixed with 45 ml of modified Tryptone Soy Broth (mTSB) (Oxoid CM 0989; Oxoid Ltd., Basingstoke, UK) and incubated at 37°C for 6 h. Afterwards, 1 ml of the enriched sample was analysed by Dynamag™–2 IMS (Invitrogen Dynal A. S., Oslo, Norway) with 0·02 ml of Captivate® E. coli O157 immunomagnetic beads (Lab M Ltd, Bury, UK) and incubated at 25°C for 30 min. After IMS, the beads were washed three times using phosphate buffered saline with 0·05% Tween 20 as wash buffer, and resuspended in 0·1 ml of the same buffer. They were then spread equally on three SMAC plates (sorbitol MacConkey agar plates (SMAC; Oxoid CM813) supplemented with cefixime (0·05 mg l-1) and potassium telluride (2·5 mg l-1) CT–SMAC), and incubated at 37°C for 18 to 24 h.
2.3. Inoculation of Milk Samples with E. Coli O157:H7
An inoculum was prepared from a fresh overnight culture (LB broth; Difco Ltd, Teddington, Surrey, UK; 18 h, 37°C, 150 rev./min) of E. coli O157:H7 [13,17] in stationary growth phase. Cells were washed and concentrated by centrifugation as described in [17]. An inoculum (1 ml) of the mixture at the appropriate dilution was added to 99 ml of each milk type and mixed thoroughly in sterilised screw–cap bottles to obtain the desired final concentration of approximately 103 CFU ml-1. All bottles of inoculated milk and uninoculated milk (control) were incubated at 4 and 20°C.
2.4. Survival and Metabolic Activity of E. Coli O157:H7
E. coli O157 cells were enumerated at 0 (immediately after inoculation), 1, 2, 4, 6, 8, 10, 12 and 14 d post–inoculation. Milk samples were serially diluted in Ringer solution (Oxoid), and serial dilutions were plated onto CT–SMAC and incubated at 37°C for 18 to 24 h. Non–sorbitol fermenting E. coli O157:H7 colonies were confirmed by agglutination with a latex test kit (Oxoid DR0620).
A parallel experiment was designed to assess variations in the activity of E. coli O157 among the different milk types (raw, laboratory–pasteurized, full-fat commercially-pasteurized, and microfiltered pasteurized). Bioluminescence of bacteria in milk was measured at 0 (immediately after inoculation), 1, 2, 4, 6, 8, 10, 12 and 14 d post–inoculation. At each time–point, a 1–ml aliquot from samples used for the enumeration study detailed above was placed into a plastic luminometer cuvette and its luminescence (RLU) was determined using a SystemSURE plus Pi-102 Luminometer (Hygiena International Ltd, UK).
2.5. Aerobic Plate Counts and PH
Aerobic plate counts (APC) were determined from uninoculated milk samples (control) at 0 (immediately after inoculation), 1, 2, 4, 6, 8, 10, 12 and 14 d. The uninoculated samples were serially diluted in Ringer solution, and serial dilutions (1:10) were plated onto plate count agar (PCA; Oxoid) and incubated at 30°C for 48 h.
Samples’ pH values were determined with a standard pH meter (Hanna instruments pH 211). Calibration was performed using two standard buffer solutions at pH 4·0 and 7·0.
2.6. Statistical Analysis
Outcomes in the experiment were changes in E. coli O157:H7 cell counts and cell activity (bioluminescence), aerobic plate counts, and pH values during the 14 d incubation period. Log (y+1) transformation was performed on E. coli O157:H7 cell counts and cell activity, aerobic plate counts, which together with pH data were subjected to ANOVA tests and Tukey's test with significance at p<0·05 using SPSS 18·0 software (SSPS Inc, Chicago, Illinois, USA).
3. Results and Discussion
3.1. Screening Milk Samples for E. Coli O157:H7
No E. coli O157:H7 was detected by the IMS method in any of the milk samples before inoculation.
3.2. Survival and Metabolic Activity of E. Coli O157:H7
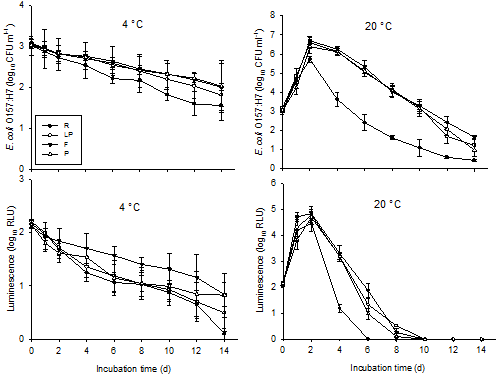
Survival and metabolic activity of E. coli O157:H7 at both 4 and 20°C are shown in Fig. 1. At 4°C, E. coli O157:H7 populations declined steadily and continuously by 1·0-1·5 log10 CFU ml-1in all samples over 14 d incubation. While log cell count reduction was greatest in raw milk (1·5 log10 CFU ml-1), between-sample variations in survival of E. coli O157:H7 were not significant between all samples at this temperature (p>0·05). Metabolic activity of E. coli O157:H7 continuously and steadily reduced (by 1·3–2·07 log10 RLU) over the 14 d, with activity in raw milk diminishing near to zero. Cell activity in the microfiltered milk was significantly higher than that in raw milk (p<0·05), while no significant difference was seen among laboratory-pasteurized, commercially-pasteurized and raw milk (p>0·05).
At 20°C, E. coli O157:H7 cell counts in all milk samples showed a dramatic initial increase, peaking at day 2 (2·7-3·6 log10 CFU ml-1), then progressively declined until the end of the 14 d incubation. Cell counts in raw milk samples decreased most (about 2·5-log cell count reduction using day 0 as baseline) and the count reduction was significantly higher (p<0·001) in raw milk compared with pasteurized samples. Counts did not statistically differ between the different types of pasteurized milk. Further ANOVA tests revealed that temperature was a significant factor moderating survival in all samples, with higher environmental temperatures leading to higher pathogen counts (p<0·001). Metabolic activity of E. coli O157:H7 at 20°C in all milk samples increased significantly on day 1, which continued to rise and peak (2·3–2·75 log10 RLU) at day 2. Cell activity dropped significantly afterwards in all samples, reaching zero in raw milk at day 6 and at day 10 in pasteurized milk samples (Fig. 1).
4. Conclusion
Pasteurized and unpasteurized milk may be contaminated with E. coli O157:H7 when inadequate farm hygiene measures (milking and milk handling) are present or post-pasteurization contamination occurs. Given the low infective dose of E. coli O157:H7 [18] and the association of milk with past infections, it is important to understand the behaviour of the organism in dairy products. Whilst others have previously studied changes in numbers of the organism in dairy products (e.g. [2], [10], [19]), this is the first study to concurrently monitor the pathogen's metabolic activity. Given the association between metabolic activity and infectivity, this paper presents novel findings of interest to dairy microbiology and food safety.
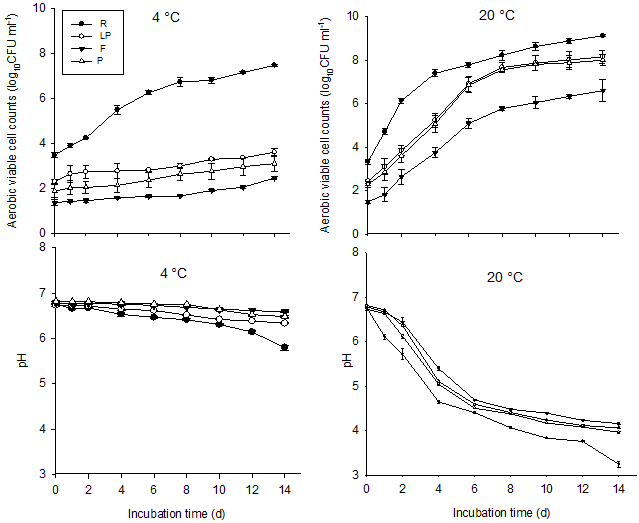
The present study confirmed that temperature is an important factor that influences the survival and activity of E. coli O157:H7. We observed that E. coli O157:H7 could not grow under refrigeration conditions in any type of milk, which was largely consistent with results from previous studies on a limited range of milk types [2]. Previous studies have recommended that milk be kept at ≤5°C as even at 7°C, E. coli O157 can grow at a significant rate [11]. Whilst other studies have also found the organism to survive and proliferate at room temperatures [2], [18], this study additionally revealed a corresponding increase in the pathogen's metabolic activity at elevated temperatures.
E. coli O157:H7 numbers and metabolic activity consistently decreased at a greater rate in raw milk than in the three types of pasteurized milk. Greater APC values were recovered from raw milk and this is expected to result in elevated competition with, and/or antagonism against the pathogen, as reported elsewhere [1], [20]. Storage of raw milk at 20°C also reduced pH considerably, most probably due to lactic acid production by the elevated counts of background micro–organisms [21]. Acidic conditions (pH<3·5, Fig. 2) are likely be detrimental to survival of E. coli O157:H7; however it should be noted that the pathogen was not found to be eliminated at these low pH values, consistent with previous studies that show its acid resistance and adaptation in acidic environments [22,23]. In addition, raw milk may also contain several compounds with bioactive components (e.g. lactoferrin, lactoperoxidase and lysozyme) that can reduce or eliminate populations of pathogenic bacteria; however these will be lost during heat treatment [24].
To conclude, examining the role of incubation temperature has practical significance in understanding how E. coli O157:H7 and other aerobic cells behave in the food chain, from retailer fridge storage to the consumer home where the greatest risk of human infection occurs. We have shown that allowing contaminated milk to reach room temperature for even a space of 2 h can induce a transient proliferation of E. coli O157:H7 numbers and metabolic activity. Although pasteurization represents an effective measure to reduce pathogenic risks and improves the microbial quality of milk, consistent hygiene quality standards must be observed both pre- and post-pasteurization to guard against any possible pathogen and spoilage microorganisms.
References
- BARBANO, DM, MA, Y & SANTOS, MV. (2006). Influence of raw milk quality on fluid milk shelf life. Journal of Dairy Science, 89, E15–E19.
- WANG, GD, ZHAO, T & DOYLE, MP.(1997). Survival and growth of Escherichia coli O157:H7 in unpasteurized and pasteurized milk. Journal of Food Protection, 60, 610–613.
- VERRAES, C, VLAEMYNCK, G, VAN WEYENBERG, S, DE ZUTTER, L, DAUBE, G, SINDIC, M, UYTTENDAELE, M&HERMAN, L. 2015. A review of the microbiological hazards of dairy products made from raw milk. International Dairy Journal. 50, 32-44.
- RILEY, LW, REMIS, RS, HELGERSON, SD, MCGEE, HB, WELLS, JG, DAVIS, BR, HEBERT, RJ, OLCOTT, ES, JOHNSON, LM, HARGRETT, NT, BLAKE, PA & COHEN, ML. (1983). Hemorrhagic colitis associated with a rare Escherichia coli serotype. New England Journal of Medicine, 308, 681–685.
- GARVEY, P, CARROLL, A., McNAMARA, E. & McKEOWN, P.J. 2016. Escherichia coli transmission in Ireland: a review of notified outbreaks, 2004–2012. Epidemiology and Infection, 144, 917-926.
- DUNCAN, SE & HACKNEY, CR (1994). Relevance of Escherichia coli O157:H7 to the dairy industry. Dairy, Food and Environmental Sanitation, 14, 656–660.
- ADAMS, NL, BYRNE, L, SMITH, GA, ELSON, R; HARRIS, JP, SALMON, R, SMITH, R, O'BRIEN, SJ, ADAK & GKJENKINS, C. 2016. Shiga Toxin-Producing Escherichia coli O157, England and Wales, 1983-2012. Emerging Infectious Disease, 22, 590-597.
- HUSSEIN, HS & SAKUMA, T. (2005). Prevalence of Shiga toxin producing Escherichia coli in dairy cattle and their products. Journal of Dairy Science, 88, 450–465.
- GOH, S, NEWMAN, C, KNOWLES, M, BOLTON, FJ, HOLLYOAK, V & RICHARDS, S (2002). Escherichia coli O157 phage type 21/28 outbreak in North Cumbria associated with pasteurized milk. Epidemiology and Infection, 129, 451–457.
- MAREK, P, NAIR, MKM, HOAGLAND, T & VENKITARAYANAN, K. (2004). Survival and growth characteristics of Escherichia coli O157:H7 in pasteurized and unpasteurized Cheddar cheese whey. International Journal of Food Microbiology, 94, 1–7.
- HEUVELINK, AE, BLEUMINK, B, VAN DEN BIGGELAAR, FLAM, TE GIFFEL, MC, BEUMER, RR & DE BOER, E. (1998). Occurrence and survival of verocytotoxin–producing Escherichia coli O157 in raw cow's milk in the Netherlands. Journal of Food Protection, 61, 1597–1601.
- JAWHARA, S & MORDON, S. (2004). In–vivo imaging of bioluminescent Escherichia coli in a cutaneous wound infection model for evaluation of an antibiotic therapy. Antimicrobial. Agents and Chemotherapy, 48, 3436–3441.
- RITCHIE, JM, CAMPBELL, GR, SHEPHERD, J, BEATON, Y, JONES, D, KILLHAM, K & ARTZ, RRE. (2003). A stable bioluminescent construct of Escherichia coli O157:H7 for hazard assessments of long–term survival in the environment. Applied and Environmental Microbiology, 69, 3359–3367.
- WILLIAMS, AP, GORDON, H, JONES, DL, STRACHAN, NJC, AVERY, LM & KILLHAM, K. (2008ª). Leaching of bioluminescent Escherichia coli O157:H7 from sheep and cattle faeces during simulated rainstorm events. Journal of Applied Microbiology, 105 1452–1460.
- WILLIAMS, AP, MCGREGOR, KA, KILLHAM, K & JONES, DL. (2008b). Persistence and metabolic activity of Escherichia coli O157:H7 in farm animal faeces. FEMS Microbiology Letters, 287, 168–173.
- THORN, CE, QUILLIAM, RS, WILLIAMS, AP, MALHAM, SK, COOPER, D, REYNOLDS, B & JONES, DL.(2011). Grazing intensity is a poor indicator of waterborne Escherichia coli O157 activity. Anaerobe, 17, 330–333.
- AVERY, L.M., KILLHAM, K. & JONES, D.L. (2005). Survival of E–coli O157: H7 in organic wastes destined for land application. Journal of applied microbiology 98, 814–822.
- CHART, H. (2000). VTEC enteropathogenicity. Journal of Applied Microbiology Symposium Supplement 88 12S–23S [OpenURL Query Data] [Google Scholar]
- MAMANI, Y, QUINTO, EJ, SIMAL–GANDARA, J & MORA, MT. (2003). Growth and survival of Escherichia coli O157:H7 in different types of milk stored at 4°C or 20°C. Food Microbiology and Safety, 68, 2558–2563.
- ELWELL, MW & BARBANO, DM (2006). Use of microfiltration to improve fluid milk quality. Journal of Dairy Science, 89, 10–30.
- KUIPPERS, OP, BUIST, G & KOK, J. (2000). Current strategies for improving food bacteria. Research in Microbiology, 151, 815–822.
- LEYER, GJ, WANG, LL & JOHNSON, EA. (1995). Acid adaptation of Escherichia coli O157:H7 increases survival in acidic foods. Applied and Environmental Microbiology, 61, 3752–3755.
- CARTER, MQ, BRANDL, MT, LOUIE, JW, KYLE, JL, CARYCHAO, DK, COOLEY, MB, PARKER, CT, BATES, AH & MANDRELL, RE. (2011). Distinct acid resistance and survival fitness displayed by curli variants of enterohemorrhagic Escherichia coli O157:H7. Applied and Environmental Microbiology, 77 3685–3695.
- INTERNATIONAL DAIRY FEDERATION (IDF). (1991) Detection and Confirmation of Inhibitors in Milk and Milk Products. IDF - Bulletin No. 258. Brussels, Belgium: IDF.

 (R. A. Lahmer)
(R. A. Lahmer)