Optimization of Medium Components for Improving the Antifungal Activity from Pseudomonas protegens XL03
Feifei Wang, Qizhong Zhang*
Institute of Hydrobiology, Jinan University, Engineering Research Center of Tropical and Subtropical Aquatic Ecological Engineering Ministry of Education, Eutrophication and Red Tide Prevention of Guangdong Higher Education Institutes, Guangzhou, People’s Republic of China
Email address

(Qizhong Zhang)
*Corresponding author
Citation
Feifei Wang, Qizhong Zhang. Optimization of Medium Components for Improving the Antifungal Activity from Pseudomonas protegens XL03. American Journal of Microbiology and Biotechnology. Vol. 3, No. 4, 2016, pp. 29-35.
Abstract
Pseudomonas protegens XL03 showed the antifungal activity against Saprolegnia sp., and its cell-free disruption supernatant (CFDS) inhibited the hyphal growth with an antifungal zone diameter of 19.34±1.20 mm. In order to improve the production of the antifungal compounds which resided in intracellular, Plackett-Burman design (PBD) and Response surface methodology (RSM) were applied to optimize the medium of the strain XL03. Firstly, the process parameters that significantly affected the antifungal compounds production (tryptone, glucose and NaCl) were identified by PBD; Subsequently, the optimum levels of these significant parameters were determined using RSM, which showed as follows: tryptone (7.50 g/L), glucose(8.79 g/L) and NaCl(1.27 g/L); Finally, the experimental validation was conducted under the optimal conditions,and the antifungal activity (antifungal zone diameter) increased about 4 mm in comparison with that on the un-optimized medium.
Keywords
Pseudomonas sp., Saprolegnia sp., Antifungal Activity, Optimized, PBD, RSM
1. Introduction
Saprolegniasis is a widespread disease in freshwater aquaculture, and causes a huge economic losses in aquaculture industry. The pathogens, Saprolegnia spp., have a ubiquitous distribution [1]. Saprolegnia spp. produced a large number of infectious spores at low temperatures [2]. Fish and fish eggs were commonly parasitized by the spores. Fungal pathogens infected the surface of the fish’s body, thus caused epidermal damage and cellular necrosis, and finally resulted in diseased fish death.
In the aquaculture industry, chemical drugs were mainly used to prevent and cure the fungal infection with their effectiveness and convenience. Malachite green was extremely effective for control of saprolegniasis, and was widely used as a therapeutic agent in aquaculture in past decades [3]. However, it has been banned for usage in fish food around the world because of its potential carcinogenicity, mutagenicity and teratogenicity [4]. Meanwhile, other chemicals, such as bronopol, sodium chloride,potassium permanganate, hydrogen and peroxide had been applied to control of saprolegniasis [1, 5], but these chemicals have no good efficacy for treatment of saprolegniasis. Besides all, a large amount of chemical drugs resulted in the environmental pollution and residues in edible aquatic animals, and were further harmful to human health. Therefore, there is an urgent need to find a safe and effective agent for controlling saprolegniasis.
Recently, microbiological control, which exploited the interaction of microorganisms to inhibit the growth of pathogens, had received more and more attention [6]. Some anti-Saprolegnia bacteria strains were discovered,such as Aeromonas sp., Serratia marcescens, Bacillus subtilis, B. amyloliquefaciens and Streptomyces sp. [6, 7]. Moreover, the saprolegniasis of Bidyanus bidyanus was successfully treated with the strain A199 [8]. This provided the feasibility of microbiological agents to prevent and cure the saprolegniasis.
In this study, the strain XL03 exhibited the efficient antifungal activity against Saprolegnia sp., and the antifungal compoundsresided in the cytoplasm. In order to improve the production of the antifungal compounds, statistical methods have been applied for optimization of fermentation medium for the maximum antifungal activity.
2. Materials and Methods
2.1. Tested Strains and Media
Saprolegnia sp. was isolated from the diseased fish tissue, and cultured on the potato dextrose agar (PDA) medium at 25°C. To obtain the fresh spore suspension, the Saprolegnia hyphae were placed into the Erlenmeyer flask with sterile water and were incubated at 25°C for 48 h, and quantified by Hu’s method [9]. All the fungal materials stored at 4°C for usage.
Pseudomonas protegens XL03 was isolated with the gradient dilution method from sediment of Baimang River in Xili, Guangzhou, China. It was cultured in Luria Bertan (LB) broth medium (1% tryptone, 0.5% yeast extract, 1% NaCl) at 28°C in an incubator shaker for 48 h, and its antifungal activity was evaluated with the following methods.
2.2. Antifungal Activity Assay of CFDS in vitro
The fermentation broth wascentrifuged at 5,974 g for 15 min at 4°C, and the cells were collected. The pellet was rinsed with phosphate buffer saline (PBS,49.5% Na2HPO4, 50.5% NaH2PO4, 0.02 M, pH 6.8) for two times, and subjected to sonication (Ultrasonic processor, UH-950B, China)for 4 s burst at 100 W and with 6 s cool period in a total of 20 min. The sonicate solution was centrifuged at 15,777 g for 30 minat 4°C,the supernatant was filtered through 0.45 μm filters to yield theCFDS.
Antifungal activity of CFDS was determined against Saprolegnia hyphal growth using agar well diffusion method [10]. Four hyphal PDA blocks (8-mm diameter) were put around the well at about three centimeters on the PDA plate, and the well was added with CFDS (200 μL), then the PDA plates were incubated at 25°Cfor 72 h. Finally, the antifungal zone diameters were measured and recorded, and the sterile PBS used as a blank control.
2.3. Selection of Basal Medium
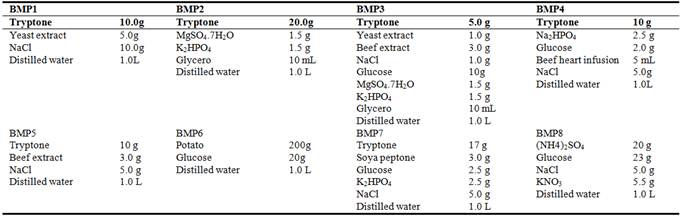
The antifungal activity of CFDS depended on growth of the strain XL03 on eight different media (Table 1). Eight kinds of culture media (200 mL) were inoculated with 2 mL of the seed culture, and incubated at 28°C, 160 rpm for 48 h. The cells were collected from the culture broth by centrifugation at 5,974 g post 48 h, and the antifungal activity of CFDS from different media was evaluated under the same conditions. The best basal medium was used for next optimization.
Table 1. Components of eight media used for selecting basal media for the strain XL03.
2.4. Screening for Medium Components Using Plackett-Burman Design (PBD)
The PBD is a statistical technique used for screening the significant variables that had an important impact on the response in the study [11]. The medium components, codes and levels of the different variables of the experimental design were listed in Table 2. The eight medium components at two levels, high (1) and low (-1) related to the 12 tests to determine their effects on the antifungal activity (Table 3). Every test was conducted in triplicates and the diameter of antifungal zone was noted as response. The variables were considered to be significant on antifungal compound production with confidence levels above 95%, and all the data analysis was conducted by Minitab 16.0 software [12].
Table 2. Variables, their codes and levels underPBD.
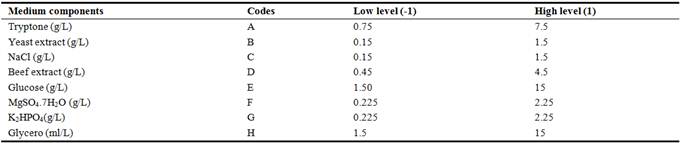
Table 3. PBD and the experimental response obtained for strain XL03. The variables of A, B, C, D, E, F, G, H represents tryptone, yeast extract, NaCl, beef extract, glucose, MgSO4.7H2O, K2HPO4, glycerol in the BMP3, respectively.-1 and 1 are code values. All experiments were done at the same time. Values are expressed as mean ± SD from three replicates.
| Trials | Variables | Diameter of antifungal zone in mm |
| A | B | C | D | E | F | G | H |
| 1 | -1 | -1 | -1 | 1 | 1 | 1 | -1 | 1 | 10.86±1.56 |
| 2 | -1 | 1 | 1 | 1 | -1 | 1 | 1 | -1 | 12.78±0.86 |
| 3 | 1 | -1 | 1 | 1 | -1 | 1 | -1 | -1 | 13.64±1.00 |
| 4 | 1 | 1 | -1 | 1 | -1 | -1 | -1 | 1 | 12.10±1.38 |
| 5 | -1 | 1 | 1 | -1 | 1 | -1 | -1 | -1 | 18.68±1.28 |
| 6 | -1 | 1 | -1 | -1 | -1 | 1 | 1 | 1 | 8.00±0.00 |
| 7 | 1 | 1 | -1 | 1 | 1 | -1 | 1 | -1 | 19.70±1.14 |
| 8 | -1 | -1 | -1 | -1 | -1 | -1 | -1 | -1 | 8.00±0.00 |
| 9 | 1 | -1 | 1 | -1 | -1 | -1 | 1 | 1 | 19.74±1.26 |
| 10 | 1 | 1 | 1 | -1 | 1 | 1 | -1 | 1 | 21.70±1.58 |
| 11 | 1 | -1 | -1 | -1 | 1 | 1 | 1 | -1 | 18.90±1.08 |
| 12 | -1 | -1 | 1 | 1 | 1 | -1 | 1 | 1 | 12.20±1.18 |
2.5. Optimization of Significant Medium Components Using Response Surface Methodology (RSM)
RSM is one of the methods to evaluate the relationship between a set of controlled experimental factors and observed results [13]. RSM was applied to determine the optimum levels of the three most significant medium components (tryptone, glucose, NaCl) through Design Expert 8.0 software in this study. The significantvariables like tryptone, glucose and NaCl were designated as A, B, and C, respectively. The low, middle, and high levels of each factor were designated as −1, 0, and 1, respectively, and shown in Table 4. The experimental plan consisted of 17 trials, and the experimental design was shown in Table 5. All the experiments were done in triplicate and the diameter of antifungal zone was taken as response. The regression analysis involving three significant independent variables (tryptone, glucose, NaCl) was performed to estimate the response function as a second order polynomial equation:
Y = 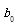 +
+ 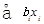 +
+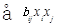 +
+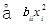 (1)
(1)
Where Y is the estimate response, b0 is constant, bi is the linear coefficient, bij is the quadratic coefficient and bii is the interaction coefficient.
The Design Expert software was applied to the regression analysis. The statistical adequacy of the model was determined through analysis of variance (ANOVA) with a confidence level of 95% (p < 0.05). The quality of the polynomial model equation was also judged by the coefficient of determination (R2) and adjusted R2. Three-dimensional surface response plots were drawn by changing two variables within the experimental range, and the other variables were held at the moderate point. An optimum level of the variables for maximum antifungal activity was obtained by regression analysis of the software.
Table 4. Variables, their codes and levels for RSM.
| Variables | Code | Level |
| | | 1.00 | 0.00 | -1.00 |
| Tryptone (g/L) | A | 7.5 | 4.125 | 0.75 |
| Glucose (g/L) | B | 15 | 8.25g | 1.5 |
| NaCl (g/L) | C | 1.5 | 0.825 | 0.15 |
Table 5. Response surface design for different medium components with three independent variables and antifungal activity in every trial.Values are expressed as mean ± SD from three replicates.
| Trials | Tryptone | Glucose | NaCl | Diameter of antifungal zone in mm |
| 1 | 0 | 1 | 1 | 22.78±1.56 |
| 2 | -1 | 0 | -1 | 22.13±0.94 |
| 3 | 0 | 0 | 0 | 23.07±1.52 |
| 4 | 1 | 0 | -1 | 21.98±1.58 |
| 5 | 0 | 1 | -1 | 22.40±1.05 |
| 6 | 0 | 0 | 0 | 22.98±1.34 |
| 7 | -1 | -1 | 0 | 21.70±0.65 |
| 8 | 0 | -1 | -1 | 22.00±1.14 |
| 9 | 0 | 0 | 0 | 22.95±0.86 |
| 10 | -1 | 1 | 0 | 22.55±1.21 |
| 11 | 1 | 0 | 1 | 22.86±1.24 |
| 12 | 1 | 1 | 0 | 22.49±1.23 |
| 13 | 1 | -1 | 0 | 22.48±1.82 |
| 14 | 0 | -1 | 1 | 22.23±1.87 |
| 15 | -1 | 0 | 1 | 22.00±0.96 |
| 16 | 0 | 0 | 0 | 22.91±2.10 |
| 17 | 0 | 0 | 0 | 22.76±1.98 |
2.6. Experimental Validation of Optimization
The statistical model and the optimization were experimentally validated by culturing the strain XL03 on the un-optimized and optimized media at 28°C for 48 h. In order to compare the antifungal activity of strain XL03 on the un-optimized and optimized media, CFDS was extracted with equal volume, and evaluated its antifungal activity under the same conditions.
2.7. Statistical Analysis
All the experiments were carried out in three replicates. Student t-test was carried out in statistical analysis system software package (SPSS 19.0), and statistically significant difference was present at p< 0.05. All experimental data were expressed as the mean ± SD (standard deviation).
3. Result
3.1. InhibitionofSaprolegnia Hyphal Growth by CFDS
CFDS displayed antifungal activity against Saprolegnia hyphal growth in plate tests. The antifungal zone diameter of 19.34±1.20mm revealed CDFS could inhibit the hyphal growth in comparison with the blank control (Figure 1).
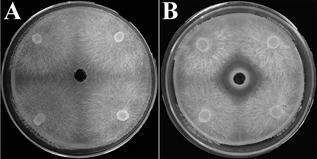
Note: CFDS, cell-free disruption supernatant; A, blank control; B, experiment group.
Figure 1. Inhibition efficacy of CFDS against Saprolegnia hyphae.
3.2. Appropriate Basal Medium
The eight kinds of media had a different effect on the antifungal activity (Figure 2). Based on the size of antifungal zone diameter, BMP3 had the highest antifungal activity, and was selected for further study.
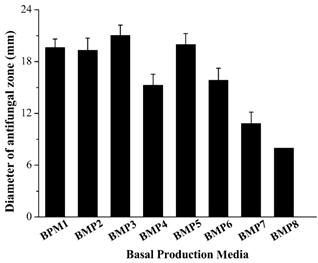
Figure 2. Effects of eight kinds of media on the antifungal zone diameter of the strain XL03.
3.3. The Most Important Medium Components for the Best Antifungal Activity
The effects of eight variables on the antifungal activity were presented in Table 6. The low p value (<0.05) indicated that tryptone and glucose were the significant impact factors. Although the p value of NaCl (0.084) is greater than 0.05, it is still an important factor in contrast to those of other factors. Therefore, tryptone, glucose and NaCl were selected as the most important components consisting of an appropriate medium for the best antifungal activity of the strain XL03.
Table 6. Statistical analysis of antifungal activity under Plackett-Burman design.
| Medium components | Effect | Standard error | t value | p-value | sequence |
| Tryptone | 5.877 | 0.6915 | 4.25 | 0.024 | 1 |
| Yeast extract | 1.603 | 0.6915 | 1.16 | 0.330 | 5 |
| NaCl | 3.530 | 0.6915 | 2.55 | 0.084 | 3 |
| Beef extract | -2.290 | 0.6915 | -1.66 | 0.196 | 4 |
| Glucose | 4.630 | 0.6915 | 3.35 | 0.044 | 2 |
| MgSO4.7H2O | -0.757 | 0.6915 | -0.55 | 0.622 | 8 |
| K2HPO4 | 1.057 | 0.6915 | 0.76 | 0.500 | 7 |
| Glycero | -1.183 | 0.6915 | -0.86 | 0.455 | 6 |
3.4. Optimization of Selected Media Components
The significant factors(tryptone, glucose, NaCl)were picked for further optimization using the RSM. The results of the seventeen experiments were shown in Table 5. The experimental data were fitted to a second-order polynomial equation by means of Design Expert 8.0 software. The response of antifungal activity was calculated by the following second-order polynomial equation:
Y=22.93 + 0.18A + 0.23B + 0.17C - 0.21AB + 0.25AC + 0.038BC - 0.37A2 - 0.26B2 - 0.32C2
Where A, B and C were the coded values oftryptone, glucose, NaCl, respectively. Statistical testing of the fitted model was checked by ANOVA, and the results were shown in Table 7. The model F value of 39.12 implied the model is significant and p value was smaller than 0.0001. The parameters values indicated that the model was suitable for this experiment. The insignificant lack of fit value of 0.09 suggested that the obtained experimental data were in good fit with the model. The predictedR2 of 0.9518 was in reasonable agreement with the Adjusted R2 value of 0.9554.
Meanwhile, in order to provide a better visualization of the statistically significant factors, the response surface 3D plots were applied to show the interactions between the important factors on the antifungal activity. The effect of tryptone and glucose on the antifungal activity was in Fig 3A. It could be seen that the antifungal activity increased with increment of tryptone when glucose was at a moderate concentration. The same trend could be seen in the effects of glucose and NaCl on the antifungal activity (Fig 3B). The antifungal activity increased with increasing concentration of tryptone and NaCl (Fig 3C). The 3D plots clearly showed that moderate level of glucose and higher levels of both tryptone and NaCl resulted in the maximum antifungal activity.
Table 7. Summary of ANOVA for response surface quadratic model analysis of variance using RSM.
| Source | Sum of Squares | df | Mean Square | F Value | p-value Prob>F | |
| Model | 2.78 | 9 | 0.31 | 39.12 | < 0.0001 | significant |
| Residual | 0.055 | 7 | 7.885E-003 | | | |
| Lack of Fit | 3.475E-003 | 3 | 1.158E-003 | 0.090 | 0.9620 | insignificant |
| Pure Error | 0.052 | 4 | 0.013 | 39.12 | < 0.0001 | |
| Core Total | 2.83 | 16 | 0.31 | | | |
| R2 | 0.9805 | | | | | |
| Adj. R2 | 0.9554 | | | | | |
| Pred. R2 | 0.9518 | | | | | |
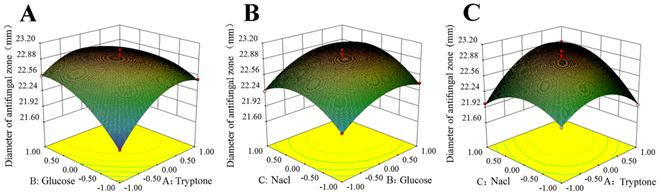
Figure 3. Response surface 3D plotes shows the relationship between the antifungal activity and the interactive effects of variables. (A) Effects of tryptone and glucose on the antifungal activity. (B) Effects of glucose and NaCl on the antifungal activity. (C) Effects of tryptone and NaCl on the antifungal activity.
3.5. Experimental Validation of Optimization
The optimumconditions for the significantvariables and the predicted values of the responses (Antifungal activity) were shown in Table 8. According to the quadratic model, the predict maximum antifungal zone diameter was 22.88 mm when the optimal values of test factors were tryptone of 7.50 g/L (coded value= 1), glucose of 8.79 g/L (coded value =0.08), and NaCl of 1.27 g/L (coded value= 0.66), respectively. Under the optimal conditions, the experimental antifungal zone diameter was 22.96±1.28 mm, which increased about 4 mm in comparison with that on the un-optimized medium. Meanwhile, the experimental value was close to the predicted value of 22.88 mm. Therefore, this indicated that the model was adequate for the study.
Table 8. Predicted and experimental values of the responses at optimum conditions. Values are expressed as mean ± SD from three replicates.
| Variables | Level | Antifungal activity (mm) |
| | Unoptimized | Optimized | Unoptimized | Optimized (Predicted) | Optimized (Experimenal) |
| Tryptone | 5.00g | 7.50g | 19.34±1.20 | 22.88 | 22.96±1.28 |
| Glucose | 10.00g | 8.79g | | | |
| NaCl | 1.00g | 1.27g | | | |
4. Discussion
Certain Pseudomonas spp. strains are plant-protecting bacteria and widespread in the rhizosphere of plant [14]. P. protegens CHA0was also called P. fluorescens CHA0 [15]. Several fluorescent Pseudomonas spp. strains protect plants from diseases caused by different soil-borne fungal pathogens, such as Gaeumannomy cesgraminis var.tritici [16], S. sclerotiorum [17] and F. graminearum [18]. Although some P. protegens strains were widely used as biological antifungal agents in the plant field, so far, they were not reported to have anti-Saprolegnia activity in aquaculture. However, P. protegens XL03 showed good antifungal activity against Saprolegnia sp. in our study, so it could be used for biological control of Saprolegnia spp. in aquaculture.
Medium components played an important role in the microbial metabolites research, and the success or failure of the whole optimization experiment was determined by the levels of carbon source and nitrogen source [19]. Therefore, in view of significance of media components and their optimum levels to the antifungal compounds of microorganisms, we have attempted to optimize medium components for improving the antifungal compounds production of P. protegensXL03. The significantmedium components for enhancing the antifungal activity were screened by PBD. The results showed thattryptone and glucose have a significantly impact on the antifungal compounds production, and the effects of tryptone and glucose on the metabolites production of Pseudomonas spp. has previously been reported [20, 21]. The optimum levels of the medium components were obtained with RSM. In present study, the antifungal zone diameter of the strain XL03 increased about 4 mmon the optimized medium in comparison with that on the un-optimized medium, so the results indicated the antifungal compounds production from the strain XL03 was enhanced. However, in the actual mass fermentation production, some factors should always be taken into consideration for the maximum production, such as incubation temperature, rotation speed, pH, oxygen demand, incubation time and inoculum level [20, 22], because these factors would also have an important impact on the antifungal compounds production.
5. Conclusions
The study discovered thatPseudomonas protegensXL03 has efficient antifungal activity against Saprolegnia sp.. Furthermore, the culture medium optimized using PBD and RSM will be useful for enhancing production of the antifungal compounds on a large scale from P. protegens XL03, and applied for controlling saprolegniasis in aquaculture.
Acknowledgements
This work was supported by Science and Technology Project of Guangdong Province (grant number:2014A020208024)and the Marine and Fishery Special Project of Science and Technology in Guangdong Province (grant number: A201301B05,A201501B09).
References
- Rach, J. J., Valentine, J. J., Schreier, T. M., Gaikowski, M. P., and Crawford, T. G. (2004): Efficacy of hydrogen peroxide to control saprolegniasis on channel catfish (Ictalurus punctatus) eggs, Aquaculture., 238: 135-142.
- Quiniou, S. M. A., Bigler, S., Clem, L. W., and Bly, J. E. (1998): Effects of water temperature on mucous cell distribution in channel catfish epidermis: a factor in winter saprolegniasis, Fish Shellfish Immun., 8: 1-11.
- Srivastava, S., Sinha, R., and Roy, D. (2004): Toxicological effects of malachite green,Aquat. Toxicol., 66:319-329.
- Zaror, L., Collado, L., and Bohle, H. (2004): Saprolegnia parasitica in salmon and trout from southern Chile,Arch. Med. Vet., 36: 71-78.
- Minor, K. L., Anderson, V. L., Davis, K. S., Van Den Berg, A. H., Christie, J. S., Lobach, L., Faruk, A. R., Wawra, S., Secombes, C. J., and Van West, P. (2014): A putative serine protease, SpSsp1, from Saprolegnia parasitica is recognised by sera of rainbow trout, Oncorhynchus mykiss, Fungal Biol., 118: 630-639.
- Hussein, M. M. A. and Hatai, K. (2001): In vitro inhibition of Saprolegnia by bacteria isolated from lesions ofsalmonids with saprolegniosis, Fish Pathol., 36:73–78.
- He, F., Xu, D. L., and Zhang, Q. Z. (2015): Screening of antagonistic bacterium strain against Saprolegnia sp. and characterization of the antifungal stability of culturebroth from the target strain, Microbiology China., 42: 1067-1074.(In Chinese)
- Lategan, M. J., Torpy, F. R., and Gibson, L. F. (2004): Biocontrol of saprolegniosis in silver perch Bidyanus bidyanus (Mitchell) by Aeromonas media strain A199, Aquaculture., 235:77-88.
- Hu, X. G., Liu, L., Chi, C., Hu, K., Yang, X. L., and Wang, G. X. (2013): In vitro screening of Chinese medicinal plants for antifungal activity against Saprolegnia sp. and Achlya klebsiana,N. Am. J. Aquacult., 75: 468-473.
- Voidarou, C., Alexopoulos, A., Plessas, S., Karapanou, A., Mantzourani, I., Stavropoulou, E., Fotou, K., Tzora, A., Skoufos, I., and Bezirtzoglou, E. (2011): Antibacterial activity of different honeys against pathogenic bacteria, Anaerobe., 17: 375-379.
- Cupul, W. C., Abarca, G. H., Carrera, D. M., and Vázquez, R. R. (2014): Enhancement of ligninolytic enzyme activities in a Trametes maxima–Paecilomyces carneus co-culture: Key factors revealed after screening using a Plackett–Burman experimental design,Electron. J. Biotechn., 17: 114-121.
- Rajeswari, P., Jose, P. A., Amiya, R., and Jebakumar, S. R. (2014): Characterization of saltern based Streptomyces sp. and statistical media optimization for its improved antibacterial activity,Front. Microbiol., 5: 753-764.
- Khajeh, M. and Sanchooli, E. (2009): Optimization of microwave-assisted extraction procedure for zinc and iron determination in celery by Box–Behnken Design,Food Anal. Method., 3: 75-79.
- Ramette, A., Frapolli, M., Saux, M. F. L., Gruffaz, C., Meyer, J. M., Defago, G., Sutra, L., and Moenne-Loccoz, Y. (2011): Pseudomonas protegens sp. nov., widespread plant-protecting bacteria producing the biocontrol compounds 2,4-diacetylphloroglucinol and pyoluteorin,Syst. Appl. Microbiol., 34: 180-188.
- Takeuchi, K., Tsuchiya, W., Noda, N., Suzuki, R., and Yamazaki, T. (2014): Lon protease negatively affects GacA protein stability and expression of the Gac/Rsm signal transduction pathway in Pseudomonas protegens, Environ. Microbiol., 16: 2538-2549.
- Haas, D. and Keel, C. (2003): Regulation of antibiotic production in root-colonizing Peudomonas spp. and relevance for biological control of plant disease, Annu. Rev. Phytopathol., 41: 117-153.
- Wang, X. F., Ke, L. F., and Zhang, J. B. (2014): Biocontrol and plant growth-promoting activity of rhizobacteria from Chinese fields with contaminated soils, Microb. Biotechn., 8: 404-418.
- Henkes, G., Jousset, A., and Lanoue, A. (2011): Pseudomonas fluorescens CHA0 maintains carbon delivery to Fusarium graminearum-infected roots and prevents reduction in biomass of barely shoots through systemic interactions, J. Exp. Bot., 62: 4337-4344.
- Cao, X. H., Cai, P., Li, F., and Wang, C. L. (2007): Medium Optmitization for Lipopeptide produced by Bacillus natto TK -1 using Response surface methodology, China Biotechnology., 27: 59-65.(In Chinese)
- Ghorbel-Bellaaj, O., Hmidet, N., Jellouli, K., Younes, I., Maalej, H., Hachicha, R., and Nasri, M. (2011): Shrimp waste fermentation with Pseudomonas aeruginosa A2: optimization of chitin extraction conditions through Plackett-Burman and response surface methodology approaches,Int. J. Biol. Macromol., 48: 596-602.
- Gaurav, K., Srivastava, R., Sharma, J. G., and Kundu, S. (2015): Statistical medium optimization for the production of cephalosporin-c acylase by Pseudomonas diminuta,J. Biochem. Technol., 6: 977-981.
- Manikandan, R., Manikandan, C. N., Sah, P., and Sah, S. (2010): Optimization of asparaginase production by Pseudomonas aeruginosa using experimental methods,Nat. Sci., 8: 1-6.

 (Qizhong Zhang)
(Qizhong Zhang) 






