Neuroprotective Effect of Hypericum thymopsis Against Chronic Exposure to Aluminum Chloride and Alzheimer's Disease
Salima Douichene, Kheira Hammadi*, Noureddine Djebli
Department of Biology, Laboratory of Pharmacognosy Api Phytotherapy, Faculty of Life and Natural Sciences, University, Mostaganem, Algeria
Email address

(K. Hammadi)
*Corresponding author
Citation
Salima Douichene, Kheira Hammadi, Noureddine Djebli. Neuroprotective Effect of Hypericum thymopsis Against Chronic Exposure to Aluminum Chloride and Alzheimer's Disease. American Journal of Pharmacy and Pharmacology. Vol. 3, No. 3, 2016, pp. 20-28.
Abstract
The effect of Aluminum chloride was investigated to describe the associated behavioral and brain modifications. We don’t know enough about the biological chemistry of chronic and sub- acute exposure to Aluminium to be able to predict its impact on human health. Although the hypothesis of a link between Aluminium and Alzheimer’s disease (AD) has been supported by several epidemiological studies. Extract of Hypericum thymopsis (HTE), a well known medicinal plant, is used for the treatment of depression, has been explored in the present study for its protective role against Aluminum neurotoxicity. This data suggests that HTE may be a candidate for application in neurodegenerative diseases such as Alzheimer’s disease. Results of this study demonstrate that Aluminiumneurotoxicity play an important role in the development of anxiety disorders, depression and memory deficit in mice, these alterations of behavioral activities can be a cause of development of Alzheimer’s disease. HTE possesses significant antioxidant activity and renders neuroprotection which was more pronounced at the dose of 200mg/ kg against Al induced neurotoxicity. Chronic administration of Hypericum thymopsis significantly improved retention in both tasks, attenuated oxidative damage, Aluminum concentration in Aluminum treated mice (p 0,05). Hypericum have neuropotective effects against Aluminum-induced cognitive dysfunction and oxidative damage.
Keywords
Aluminum, Alzheimer’s Disease, Hypericum Thymopsis Extract (HTE) Neuroprotection, Mice
1. Introduction
Aluminum (Al) is one of the most abundant metals in the earth’s crust.
It’s a light weight metal widely used by man, this element appears mainly in food products, driking water derived from both natural sources and treatment methods[1].
The human toxicological effects include encephalopathy [2], bone disease [3] and anemia [4]. Aluminum is recognized as a risk factor for neurological disorder’s, such as Parkinson’s disease, amyotrophic lateral sclerosis and Alzheimer’s disease (AD) [5].
Today, there is no cure for this neurodegenerative disease and there for it is of great interest for researchers to find new drugs that can inhibit the acetylcholin esterase and N- Methyl-D- Aspartate (NMDA)- receptor antagonist.
These drugs improve the function of still intact neurons, but do not inhibit the ongoing degenerative process leading the neuronal cell death [6]. Eighty-nine Hypericum species occur in turkey, 43 of which are endemic as recorded in the flora of Turkey.
Hypericin was first identified in hypericum species and servers as a chemotaxonomic marker of the genera [7].
Antioxidant activity of ethanol and water extracts of the flowers of hypericum was investigated. It was observed that antioxidative activities of ethanol extracts of Hypericum thymopsis are comparable with vitamin E [8].
Moreover, aqueous extracts prepared from the flowering aerial parts of the hypericum species are being used in the treatment of physiological diseases such as neurolgia, anxiety, neurosis and depression. The assess a spectrum of learning and memory functions, a battery of tests is needed. These [9, 10]. tests are selected to assess behavior and memory. The present studies are undertaken to assess the neurotoxicity of Aluminum and the neuroprotective effect of Hypericum thymopsis after chronic exposition of albinos mice.
2. Materials and Methods
Twenty four healthy adult mice weighting from 32+-4 g were obtained from Pasteur institute Algeria, they were maintained at 25+-5°C with a 12 h light/ dark cycle, and have been given a commercial pellet diet 18 g /day/ mouse (ONAB) and fresh drinking water ad libitum.
The mice were randomly divided into four groups: each group containing six mice: control group neurotoxicity and Alzheimer model, intoxicated/ Alzheimer treated groups and the control treated groups.
AlCl3 dissolved in distilled water administrated orally (10mg /kg) for the intoxicated /Alzheimer’s model groups, and intoxicated/Alzheimer’s treated groups with a D- Galactose IP (120mg/kg) for the Alzheimer’s model given for 90 days; in parallel of Hypericum thymopsis administration (200mg/kg orally) respectively for the intoxicated treated group and Alzheimer’s disease animal.
The control treated group received the same dose of Hypericum thymopsis (200mg/kg) administrated orally. Controls were treated with distilled water. Body weight was recorded daily but no significant differences were observed between the groups.
3. Behavioral Tests
Functional behavioral assessment is required as part of testing the nervous status, these guidelines apply to animals in special tests: locomotor activity, holeboared (curiosity) [11]., black and white test box (anxiety) [12]. and forced swimming (Persolt test) [13].
4. Memory Tests
The assessment of animal memory using different types of mazes has been used in neuroscience [14].
Several models have been proposed recently mainly trying to evaluate accuracy of choice between the alternatives presented in the same day of the session, instead of looking for the accumulated learning through successive days of training.
Moris water maze:
The Morris water maze is widely used to study spatial memory and learning [15]. The maze consists of circular pool (1, 2 m in diameter and 0, 47 m high) made of white plastic (9, 10). The pool was filled to a depth of 20 cm with water (24°C-25°C) that was made opaque by the addition of no toxic white paint. An escape platform (10cm in diameter), made of white plastic with a grooved surface for a better grip, was submerged 0, 5 cm under the water level.
The animal has to swim until it finds the hidden platform. The animal generally uses cues outside the maze to develop a spatial map of the environment and guide its performance. The pool was divided into four equal quadrants labeled N (north), E (east), S (south), W (west).
Their order of use was randomized daily. The time the mouse needed to find the hidden platform (latency) so that it can stop swimming was recorded. A trial was started by placing the mouse into the pool close to the rim, facing the wall of the tank into one of the four quadrants. The mice were given four days of training with four 60 seconds training trials per day. During a spatial reference memory (SRM) training, the platform was always placed in the same spatial location of the pool (NE quadrant) throughout the training period in both paradigms. During spatial working memory (SWM) training, the escape platform was placed from the edge of the pool in one of the four possible locations (designated N, S, E and W).
5. Histological Studies (Preparation of Brain Tissue)
At the end of exposure, animals were food deprived for 24h
Mice from both studies were sacrificed with an overdose of Chloral.
After the step-through avoidance test, the brains of mice were removed right after sacrifices. Removed brains were then quickly impressed in 10% neutral buffered formaldehyde for 24hours. Serial coronal paraffin section were cut at 4µm were obtained with a Leica microtome thickness for hematoxylin &eosin (H&E) 1% for 40 seconds.
6. Statistical Analysis
The experimental results were reported as means with SEM. Statistical analysis was performed using SPSS Software. Analysis of variance (ANOVA) and an LSD test were used to compare the experimental groups with the controls. One-way ANOVA P value using the post hoc Fischer’s LSD test. P-value<0.05 was considered significant.
7. Neurological Parameters
7.1. Behavioral Test
Test of locomotor activity:
The results obtained after chronic poisoning with aluminum chloride followed by treatment with Hypericum thymopsis shows hyperactivity in mice intoxicated and Alzheimer’s and Alzheimer's mice treated compared to the control model. Moreover, this activity is gradually decreased during the experimental period in all groups.
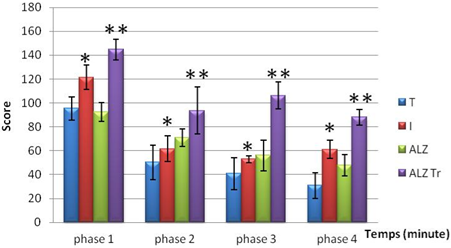
Highly significant difference (P < 0.01)
Figure 1. locomotor activity in mice intoxicated by aluminum (10 mg/kg), Alzheimer model mice poisoned by AlCl3 (10 mg/kg) with D-galactose (120 mg/kg) intraperitoneally and mice Alzheimer model treated Hypericum thymopsis (200 mg/kg) compared to control mice for 12 weeks.
7.2. Test of Curiosity
The results obtained during test curiosity showed that mice treated with the extract of Hypericum thymopsis are curious during the 1st phase of experimentation, the result is closer to that of the control group, this curiosity is decreased gradually during the following phases.
By cons mice poisoned by AlCl3, and Alzheimer mouse model poisoned by AlCl3 orally and D-galactose intraperitoneally and less exploratory than the other groups.
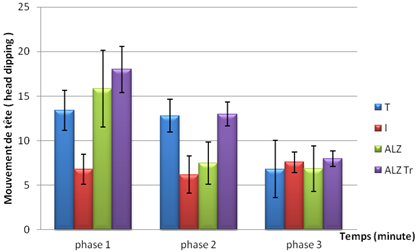
Figure 2. Test of curiosity in mice intoxicated by aluminum (10 mg/kg), Alzheimer model mice poisoned by AlCl3 (10 mg/kg) with D- galactose (120 mg/kg) intraperitoneally and mouse model of Alzheimer's disease treated with Hypericum thymopsis (200 mg/kg) compared to control mice for 12 weeks.
7.3. Test of Anxiety
The results obtained during the test of anxiety show that the residence time in the dark compartment in the experimental groups is very important for the duration of experimentation.
We note that the mouse model Alzheimer spend much more time in the dark during the third and fourth phases compared to other groups.
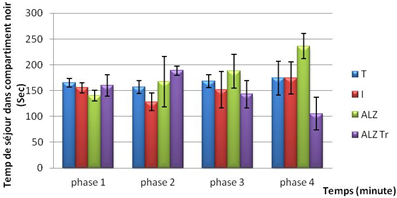
Figure 3. Test anxiety in mice intoxicated by aluminum (10 mg/kg), Alzheimer model mice poisoned by AlCl3 (10 mg/kg) with D- galactose (120 mg/kg) by IP and Alzheimer model mice treated with Hypericum thymopsis (200 mg/kg) compared to control mice for 12 weeks.
7.4. Test Cross
In this test it was observed that the intoxicated and Alzheimer model group, the treated and control mice spend more time in the hallway arm protected throughout the experiment.
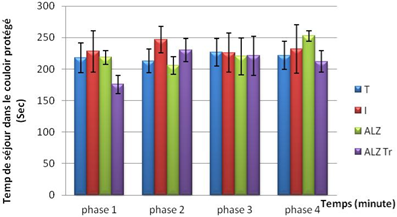
Figure 4. Test elevated plus maze in mice intoxicated by aluminum (10 mg/kg), Alzheimer model mice poisoned by AlCl3 (10 mg/kg) with D- galactose (120 mg/kg) IP and Alzheimer model mice treated with Hypericum thymopsis (200 mg/kg) compared to control mice for 12 weeks way.
7.5. The Forced Swimming Test
During the forced swim test was observed that the time recorded in the control mice treated immobility is much larger than mice poisoned by AlCl3 orally at a dose of 10mg/kg and intoxicated by AlCl3 at the same dose and D- galactose intraperitoneally at a dose of 120 mg/kg have recorded a short time.
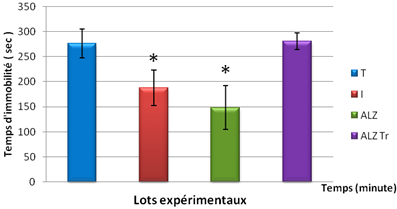
Figure 5. Test Persolt in mice intoxicated by aluminum (10 mg/kg), Alzheimer model mice poisoned by AlCl3 (10 mg/kg) with D- galactose (120 mg/kg) intraperitoneally and mouse model of Alzheimer's disease treated with Hypericum thymopsis (200 mg/kg) compared to control mice for 12 weeks.
7.6. Memory Test
8-arm radial maze
A. Spatial working memory:
In the spatial working memory test shows that the number of repeated passages in mice and treated Alzheimer model and witness higher unlike intoxicated during the first four days of learning group.
But during the fifth day, we see that the number of repeated corridors is much greater in the control group compared to other groups.
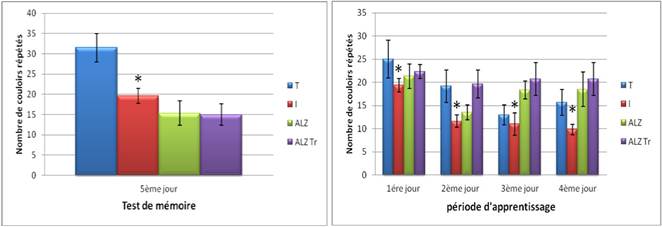
Figure 6A. Test of spatial working memory in mice intoxicated by aluminum (10 mg/kg), Alzheimer model mice poisoned by AlCl3 (10 mg/kg) with D- galactose (120 mg/kg) IP and Alzheimer model mice treated with Hypericum thymopsis (200 mg/kg) compared to control ice for 12 weeks way.
B. Non-spatial memory preferably condition:
The results obtained in testing non-spatial working memory preferably packaged show that the residence time in the arms illuminated in four groups approached ente them this time is increased in controls and Alzheimer treated with Hypericum thymopsis during the second and third tests unlike intoxicated intoxicated group and Alzheimer model.
On the fifth day, we note that the residence time in the baited arm was higher in the group Alzheimer 's and Alzheimer's compared to controls (non-significant results) Treaty by against the residence time in the intoxicated group is reduced compared to control.
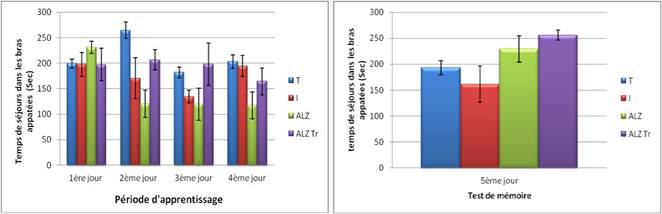
Figure 6B. Test of non-spatial memory preferably packaged in mice intoxicated by aluminum (10 mg/kg), Alzheimer model mice poisoned by AlCl3 (10 mg/kg) with D-galactose (120 mg/kg) intraperitoneally and Alzheimer model mice treated with Hypericum thymopsis (200 mg/kg) compared to control mice for 12 weeks.
C. Regardless of position:
When testing regardless of position it was observed that the treated group and model Alzheimer choose baited the control arm, this choice is decreased in the intoxicated during the first two phase’s group. On Alzheimer mouse model registered in the baited arm number is reduced during the third and fourth day of learning.
On the fifth day, it is clear that the control mice compared to choose the baited and poisoned mouse model Alzheimer arms more frequently.
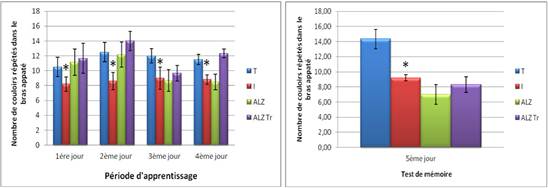
Figure 6C. Test position of distinction in mice intoxicated by aluminum (10 mg/kg), Alzheimer model mice poisoned by AlCl3 (10 mg/kg) with D- galactose (120 mg/kg) by IP and Alzheimer model mice treated with Hypericum thymopsis (200 mg/kg) compared to control mice for 12 weeks.
Morris water maze:
A. Spatial working memory (MST):
The results of the test of spatial working memory show that poisoned mouse model Alzheimer take much longer to detect the visible platform as compared to controls and intoxicated group treated during the first and second day of the experiment.
On the other hand, it was noticed that the mouse model Alzheimer take a long period to detect the platform relative to the control and treated.
On the fourth day, the mouse Alzheimer model takes a long time to detect the visible platform compared with witnesses who latency reduced progressively testing.
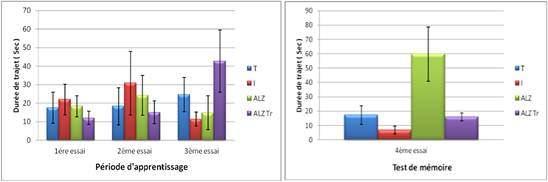
Figure 7A. Test Pool Morris ' spatial working memory (MST) "in mice intoxicated by aluminum (10 mg/kg), Alzheimer model mice poisoned by AlCl3 (10 mg/kg) with D- galactose (120 mg/kg) intraperitoneally and Alzheimer model mice treated with hypericum thymopsis (200 mg/kg) compared to control mice for 12 weeks.
B. Spatial reference memory (MSR):
During the spatial reference memory (MSR) the journey to the invisible platform is significantly higher in Alzheimer model group from the second trial until the end of the experiment.
However, we note that the journey to the invisible platform is significantly reduced in intoxicated compared to controls.

Significant difference (P < 0.05)
Figure 7B. Test Pool Morris ' spatial reference memory (MSR) "in mice intoxicated by aluminum (10 mg/kg), Alzheimer model mice poisoned by AlCl3 (10 mg/kg) with D- galactose (120 mg/kg) intraperitoneally and Alzheimer model mice treated with Hypericum thymopsis (200 mg/kg) compared to control mice for 12 weeks.
8. Results of Histological Studies
H&E staining shows that there are typical neuropathological changes in the hippocampus of intoxicated/Alzheimer’s model. In the control groups the neurons were full and arranged tightly, the nuclei were light stained. By comparison in the model group mice the cytoplasm of neuron were shrunken, the nuclei were side moved and dark stained, neurofibrillary degeneration and neurons loss were observed in hippocampus. Curcuma administration shows moderated neuropathological changes. The neurons recovered their characteristic shape, with prolonged neurofibrillary tangles (Figure 8).
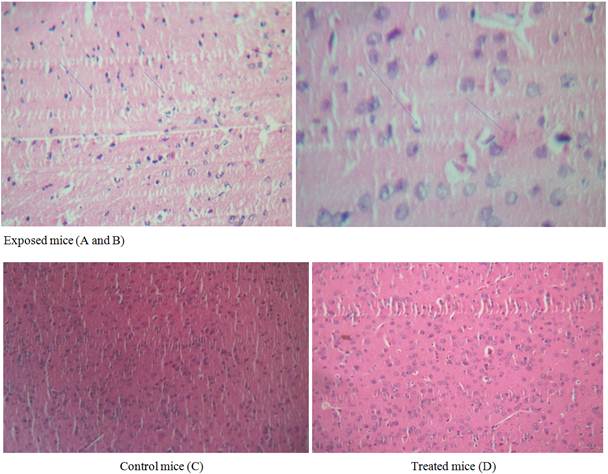
Figure 8. Microscopic study of cerebral cortex in mouse brain. Grossly (x 40). Histological sections of brain were stained with hematoxylin & eosin (H&E).
Exposed mice (A, B) to 10 mg/kg/day AlCl3 during 12 weeks. Control (C). Treated mice (D) with 200mg/kg of Hypericum thymopsis during 12 weeks.
9. Discussion
The test results show that the locomotor activity and the poisoned mouse Alzheimer model mice and mice treated with Hypericum thymopsis are hyperactive compared to control mice. These results are not consistent with the work of Golub et al [16] and Kumar et al [17] have observed that locomotor activity decreases due to the aluminum intoxication.
The test results of curiosity, which measures the exploratory behavior shown by mice reveal that poisoned mice and Alzheimer mouse model are less exploratory than the control and treated mice. This suggests that aluminum toxicity induced damage to the nervous system that result coincides with the work of Gourrier - Fréry [18]
The results obtained during the test anxiety indicate that groups of intoxicated mice treated and control spend much time in the dark. These results are not consistent with the work and Rebai Djebli et al [19]; Costall et al [20]. Who noticed that poisoned prefer the light compartment.
The arms are well protected anxiety in mice intoxicated by aluminum and the results obtained when testing at elevated cross maze show that poisoned mice and Alzheimer model mice prefer to stay in the protected arm. Similarly, the control mice and mice treated the same compartment prefer unprotected. These results are consistent with the work of Djebli Rebai [19] and who found the same result.
The forced swim test (Persolt) reveals that the immobility time of control mice and mice treated Alzheimer model is more important than the poisoned mice and Alzheimer mouse model. These results are consistent with several studies Sahin et al [21]. and Gardier Bouirin [22].
Test of 8-arm radial maze, in the event of spatial working memory, it has been shown that the number of repeated corridors is much higher in the control mice, Alzheimer model and processed compared to intoxicated mice and our results not confirm the number of errors is intoxicated higher compared to controls.
These results translate into a deficit of spatial working memory in procedural learning that is caused by an accumulation of aluminum in the brain, this result is consistent with the work of Santucci et al [23].
On the test regardless of position it was found that the control mice and mice treated Alzheimer model choosing baited arms unlike poisoned mouse.
As for the non-spatial memory test preference conditioning, mice intoxicated is widely taken time to get to the food than control mice and treated intoxicated, this result is consistent with the work of Santucci et al [23].
In both versions of the test of the Morris water maze, spatial working memory and reference it was noted that the poisoned mice and Alzheimer mouse model takes longer to find compared to mice treated and control platform. These results are consistent with the work of Luo et al [24] noted that these poisoned take much more time to find the platform compared to controls. These poisoned mouse Alzheimer model shows a deficit of spatial learning and the ability to store
10. Conclusion
In the past, aluminum was considered perfectly harmless and no one had thought to study its methods of penetration into the body and its behavior in the organs.
Today, aluminum toxicity is well established in laboratory animals and in patients with renal failure, and an epidemiological link has actually been demonstrated between aluminum exposure and neurodegenerative diseases.
In our experimental study we attempted to evaluate the antioxidant effect of Hypericum thymopsis on chronic neurotoxicity of aluminum, for a period of 12 weeks.
The neurotoxic effect of aluminum has been proven by a number of neurological disorders that result in a decrease in body weight, disturbances stereotyped behavior, exploratory activity (locomotor activity test holes) and anxiety and a memory deficit and proved by the results of the test of the Morris water maze learning
References
- Yokel, R.A. and P.J. McNamara, 2001. Aluminum toxicokinetics: an updated minireview.Pharmacol. Toxicol; 88:159-167.
- Alfrey, AC; G.R. LeGendre and W.D.Kaehny, 1976. The dialysis encephalopathy syndrome. posiible aluminum intoxication.N.Engl.J.Med; 294:184-188.
- Klein, G.L; 1993.Aluminum and hepatobiliary complications of total parenteral nutrition. Gastroenterology, 10: 1583-1584.
- Ward, M.K; T.G.Feest, H.A. Ellis, I.S.Parkinson and D.N. KERR, 1978. Osteomalacic dialysis osteodystrophy: Evidence for a water-borne aetiological agent, probably aluminum.Lancet, 22: 841-845.
- Short, A.I.K., R.J. Winney and J.S. 1980. Robson, Reversible microcytic hypochromic anaemia in dialysis patients due to aluminum intoxication.Proc. Eur.Dial.Transplant.Assoc; 17:226-233.
- Yamada K, Nabeshima T. Animal models of Alzheimer’s disease and evaluation of anti-dementia drugs. Pharmacol Therapeut 2000; 88: 93-113.
- Robson, N. "Studies in the genus Hypericum L. (Clusiaceae). 1 Section 9. Hypericum sensu lato (part 3): subsection 1. Hypericum series 2. Senanensia, subsection 2. Erecta and section 9b. Graveolentia. 2006" Systematics and Biodiversity 4:19-98.
- Spiteller M, Ozen T, Smelcerovic A, Zuehlke S, Mimica-Dukic N. Phenolic constituents and the in vitro antioxidant activity of the flowers of H. venustum. Fitoterapia 79(3):191-193. 2008.
- Green, R.J. and M.E. Stanton, 1989. Differential ontogeny of working memory and reference memory in the rat. Behav.Neurosci; 103:98-105.
- Schenk, F. and R.G. Morris, 1985. Between components of spatial memory in rats after recovery from the effects of retrohippocampal lesions. Exp. Brain Res; 58:11-28.
- Boissier, J.R. and P. Simon, 1962. The exploration reaction in the mouse. Preliminary note. Therapie; 17:1225-1232.
- Costall, B., M.E. Kelly, R.J. Naylor and E.S. Onaivi, 1989. The effects of ondansetron (GR38032F) in rats and mice treated subchronically with diazepam.Pharmacol. Biochem. Behav; 32:777-785.
- Persolt, R.D; A. Bertin and M. Jalfre, 1977. Behavioral despair in mice: a primary screening test for antidepressants; Arch.Int. Pharmacodyn. Ther; 229:327-336.
- Pan R, Qiu S, Lu DX, Dong J. "Curcumin improves learning and memory ability and its neuroprotective mechanism in mice" Chin Med J (Engl). 2008 May 5; 121(9):832-9.
- Morris, R.G.and A.D.Baddeley, 1988. Primary and working memory functioning in Alzheimer-type dementia. J. Clin. Exp. Neuropsychol; 10: 279-296.
- Golub, M.S.;Keen CL., Gershwin ME. Neurodevelopemental effect of aluminium in mice: forstering studies. Neurotoxicol teratol 14: 177-182 1992.
- Anil Kumar; Samrita Dogra; Atish Prakash "protective effect of curcumin (curcuma longa), against aluminium toxicity: possible behavioral and biochemical alteration in rats. Behavioural Brain research 205 (2009) 384-390.
- Gourier-Fréry C, Fréry N, Berr C, CordierS, Garnier R, Isnard H, et al. Aluminium. Quels risques pour la santé? Synthèse des études épidémiologiques. Volet épidémi-ologique de l’expertise collective INVS-Afssa-Afssaps. InstitutdeVeilleSanitaire; novembre2004, 270p.
- Rebai Ouafa and Nour Eddine Djebli "Chronic Exposure to Aluminum Chloride in Mice: Exploratory Behaviors and Spatial Learning" Advances in Biological Research 2 (1-2): 2008.
- Costall, B., M.E. Kelly, R.J. Naylor and E.S. Onaivi, 1989. The effects of ondansetron (GR38032F) in rats and mice treated subchronically with diazepam. Pharmacol.Biochem. Behav., 32: 777-785.
- Sahin, G., Varol, I. and Temizer, A., Determination of aluminum levels in the kidney, liver and brain of mice treated with aluminumhydroxide, Biol. Trace. Elem. Res., 41:129-135(1994).
- Gardier, A.M. and M. Bourin, Appropriate use of "knockout" mice as models of depression or models of testing the efficacy of antidepressants. Psychopharmacology, 153: 393-4. 2001.
- Santucci D., Judith Rankin., Giovanni Laviola., Luiji Aloe.,Section of behavioral pathophysiology, Lab.Di Fisiopatologia di Organo edi sistima, Istitute superior di sanita, vial Regina Elena 299,1-00161, Roma, Ilaly b Institue of cell, Animal and population Biology, King’s buildings, west Mains road, Edinburgh EH9 3JT, UKc Istituto di Neurobiologia, NR, vial Carlo Marx 15,1-00155, Roma, Italy (5 March 2003).
- Luo Y, Nie J, Gong Q.H, Lu Y.F, Wu Q, Shi J.S:Protective effcts of icarin against learning and memory deficits induced by aluminum in rats; Clin. Exp. Pharmacol. Physiol. 2007 Aug; 34(8): 792-795.

 (K. Hammadi)
(K. Hammadi) 












