Clinical and Therapeutic Potentials of the Ethylacetate-Soluble Constituents of Monodora Myristica Seed Isolated by Preparative Thin Layer Chromatography (PTLC)
Ezeudo Ewuziem Nwaozuzu1, *, Godwin Chukwu Ebi2
1Pharmacy Department, Federal Medical Centre, Owerri, Imo State, Nigeria
2Pharmaceutical Chemistry Department, Faculty of Pharmaceutical Sciences, University of Nigeria, Nsukka, Enugu State, Nigeria
Email address

(E. E. Nwaozuzu)
*Corresponding author
Citation
Ezeudo Ewuziem Nwaozuzu, Godwin Chukwu Ebi. Clinical and Therapeutic Potentials of the Ethylacetate-Soluble Constituents of Monodora Myristica Seed Isolated by Preparative Thin Layer Chromatography (PTLC). American Journal of Pharmacy and Pharmacology. Vol. 3, No. 2, 2016, pp. 6-13.
Abstract
Monodora myristica (fam. Annonaceae) is one of the plants used in the treatment of malaria in Ibo-Nigeria alternative medicine. It has also been claimed to possess antimicrobial and antifungal properties. The Monodora plant is a perennial ornamental tree with a height of up to 30m high, dense foliage and spreading crown. It flowers from September to April at which time the new leaves appear. The fruits are produced between April and September. They are about 15cm in diameter, green, round, woody and are suspended in a long stalk. The pulp is white and contains numerous seeds of about 2.5cm long. Previous preliminary studies on the plant’s seed by the authors showed that the ethylacetate-soluble fraction of its methanolic extract possess significant and higher antimicrobial properties than the ethylacetate-insoluble fraction. The study was therefore designed to evaluate the antimicrobial properties and potential therapeutic applications of the the ethylacetate-soluble constituents of the plant’s seed. Monodora myristica seeds were sun-dried, milled and extracted by cold maceration with 95% methanol. This methanolic extract was further fractionated with ethylacetate to obtain an ethylacetate-soluble fraction and an ethylacetate-insoluble fraction. The constituents of the ethylacetate-soluble fraction were then isolated by PTLC using pre-coated alumina and silica gel GF254 plates. Penicillin G, chloramphenicol and nystatin were used as controls for the evaluation. Solutions of the ethylacetate-soluble constituents together with solutions of the controls were standardized to 10mg/ml solution in dimethyl sulphoxide solutions. These standardized solutions of the extracts including the controls were then evaluated for antimicrobial activity against some gram-positive bacteria (Bacillus subtilis, Staphyllococcus aureus), gram-negative bacteria (Klebsiella pneumoniae, Pseudomonas aeruguosa, Salmonella typhi, Escherichia coli), the yeast (Candida albicans) and the mould (Aspergillus niger) using the agar disk method. The results showed that the most active bands were bands 4/5, 9, 17 and 19. These bands showed significant antimicrobial activities mostly against K. pneumonia, and E. coli. The minimum inhibitory concentrations (MICs) showed that band 9 was the most potent against K. pneumonia while band 19 was most potent against P. aeruginosa. The activity
Keywords
Monodora myristica, Antimicrobial Properties, Preparative Thin Layer Chromatography (PTLC), Ethylacetate Fractions, Ibo-Nigeria Folkloric Medicine
of these different PTLC bands of M. myristica seed lends credence to many of its uses in Ibo-Nigeria folkloric medicine.
1. Introduction
M. myristica commonly known as ‘Ehuru’ in Eastern Nigerian is an edible plant most commonly found in the evergreen forests of West Africa. Its seed has an odour similar to that of nutmeg and that’s probably why it is also called African nutmeg. The seeds are the most important part of the tree though almost every part of the tree is economically important.
In Central African Republic, the seeds are used to treat headaches and hypertension while in Ibo-Nigerian medicine they are used as condiments and as a spice in postpartum tonics. M. myristica possess anti-sickling properties and its berry possesses diuretic properties and is used for mild fever and as antiseptic. The kernel is also used to prepare soup as a stimulant to relieve constipation and control passive uterine haemorrhage in women after child birth (Nwozo et al, 2015). It has been reported to possess high antifungal properties (Tatsadjieu et al, 2003) as well as many other antimicrobial properties, hence its variety of uses in Ibo-Nigeria folkloric medicine (Okpala, 2015; Owokotomo & Ekundayo, 2012). M. myristica plant is widely used to relieve toothache (the root) as well as treat dysentery. The roasted and pulverized seeds have reportedly been used to treat unspecified skin diseases suggesting germicidal or antiseptic potential while the crushed seed can be used as an insecticide (Eze-Steven et al, 2013). There have also been reports of its use in the treatment of stomach-aches, febrile pains, eye diseases and haemorrhoids as well as a spicing agent for African and Continental cuisines in Nigeria due to its aromatic flavour (Owokotomo & Ekundayo, 2012).
Though many spices have been used by many cultures for ages mostly for seasoning purposes, yet their nutritional, phytochemical, antioxidant, preservative and medicinal potentials have also been observed (Enabulele et al, 2014), hence this serial evaluation of the antimicrobial/therapeutic potentials as well as the phytochemistry of M. myristica seed by the authors. The phytochemicals that have been observed in M. myristica include glycosides, sterols and triterpenoids, aldehydes and unsaturated compounds (Nwaozuzu & Ebi, 2015).
Meanwhile, the many and diverse medicinal properties of M. myristica seed could lie in the many different phytochemical constituents of the seed which the authors have separated in this study. However the study focused more on the antimicrobial properties of these constituents. The other constituents which did not show antimicrobial properties could also possess other medicinal properties like the ones the plant has been claimed to possess by different cultures across the globe as enumerated above. The authors therefore recommend more investigations into the much other medicinal potential of this utility medicinal plant by researchers all over the world.
2. Materials
2.1. Plant Materials
This consisted of the seeds of M. myristica. They were collected in September at Nsukka in Enugu state of Nigeria by Mr Paulinus Ugwu and Mr J. E Ekekwe both of Botany department of University of Nigeria, Nsukka. They were then prepared by cutting, sun - drying and milling. The powdered forms were then used in the experiments.
2.2. Reagents
Sulphoric acid, Chloroform, Ammonia solution, Ferric chloride, Fehling’s solution 1 and 11, Ethylacetate, Hydrochloric acid, Glacial acetic acid, Aluminium chloride, Ethanol, Bromine water, Mayer’s reagent, Distilled water, Sodium hydroxide, Tollen’s reagent, 2, 4 – dinitrophenylhydrazine, Acetic anhydride, acetic acid, silica gel GF254.
2.3. Solvents
Methanol, Ethylacetate, Methylethylketone (MEK), MEK / Hexane, Dimethyl sulphoxide (DMSO), Chloroform and Ethanol.
2.4. Instrumentation
Uniplan TLC spreader, Chromatographic tank, Aluminium plates, Silica plates, Seperating funnel, Evaporating dish, Rotary evaporator, Water bath, Capillary tubes, Test tubes, Conical flasks, Measuring cylinders, Beakers, Pipettes, Funnels, Filter papers, Weighing balances, Glass chromatoplates, UV lamp, Bunsen burner and Spatula.
2.5. Microbiological Materials
Broth cultures of test organisms [gram-positive bacteria (B. subtilis, S. aureus), gram-negative bacteria (K. pneumonia, P. aeruginosa, S. typhi, E. coli), the yeast (C. albicans) and the mould (A. niger)], Sterile Petri dishes, Sterile Cork borer, Sterile Forceps, Inoculation loop, Incubator, Autoclave, Indelible Marker, Lighter, Nutrient agar, Paper strip, Penicillin G, Chloramphenicol and Nystatin.
3. Methods
3.1. Extraction and Fractionation of Monodora Myristica
The extraction was carried out by cold maceration using 95% methanol. The powered drug was cold macerated with the solvent for 24 hours. The extract was filtered off. The process repeated several times until the constitutions were completely extracted, indicated by the colorlessness of the extraction solvent. The extract was then evaporated to dryness under reduced pressure, using the rotary evaporator. The extract was then washed with several portions of ethylacetate to get the ethylacetate-soluble and ethylacetate-insoluble fractions. The solution of the ethylacetate-soluble fraction was evaporated to dryness with the rotary evaporator.
3.2. Analytical TLC of the Fractions
Analytical TLC of the ethylacetate-soluble and insoluble fraction was carried out using pre-coated alumina and silica gel GF254 plates. The solutions of the ethylacetate-soluble and ethylacetate-insoluble fractions were prepared in dimethylsulphoxide. About 3 drops of each solution was placed about 8mm from one end of the plate. On drying the plate was gently dipped into a small chromatographic tank previously equilibrated with the developing solvent system for development. After the solvent had moved a reasonable distance, the plate was removed, allowed to dry, viewed and marked under both the UV (254 and 365nm) light.
3.3. Isolation of Ethylacetate-Soluble Fraction by PTLC
3.3.1. Preparation of PTLC Plates
Five glass plates were washed using detergent solution and allowed to dry. The plates were arranged in a row in the spreading rail and cleaned with acetone-soaked cotton wool. 40g of the silica was mixed with distilled water in the ratio of (1:2) and vigorously shacked in a conical flask to form slurry. This was poured into the spreading rail and the spreader moved to coat the plates to 0.7mm thickness. The coated plates were allowed to air-dry at room temperature and then activated at 110°C for 1hr before being used.
3.3.2. Isolation of Etac Soluble Fraction Constituents
The ethylacetate- soluble fraction was then separated by applying the solution as a band about 4cm from the edge of the glass plate using a capillary tube. The spotted band was allowed to dry and the plate gently placed into the developing solvent system, Hexane-MEK (3:1). The tank was securely closed and the plate developed by allowing the solvent to run up the spotted plate. After the solvent front had moved to the edge of the plate, it was removed from the tank and marked under UV-light. The different bands were crapped out into different bottles and labeled. The entire isolation process is represented in figure 1 below.
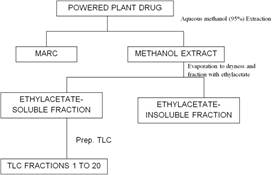
Figure 1. Flow chart for isolation.
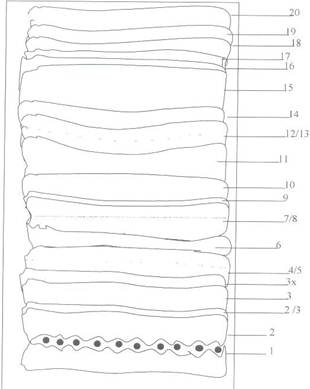
Figure 2. PTL Chromatogram of ETAC-Soluble fraction of M. myristica seed on Silica gel (GF254) using Hexane-Mek (3:1) as solvent system.
3.3.3. Standardization of the PTLC Isolates
Some quantities of the absorbed bands were desorbed with methanol to obtain the methanol extract of the bands. Some volume of the methanol extract was introduced into a small round bottom flask of known weight and the solvent completely driven off under reduced pressure using rotary evaporator. The weight of the solid sample was then determined and the desired volume of dimethyl sulphoxide (DMSO) used to form the standard solutions.
3.4. Determination of Microbial Sensitivity to the PTLC Fractions
Clean, dry Petri dishes and nutrients agar medium (Sabouraud’s dextrose agar) in bijou bottles were sterilized in an autoclave at 121°C for 15 minutes. The content of each bottle (about 20ml) was aseptically poured into each Petri dish. 0.1ml of an appropriate dilution of the desired microbial culture/suspension was added to the molten agar and the plate rotated gently to ensure even distribution of the micro-organism and then allowed to set. Using a sterile cork borer of diameter 6mm, cups were made on the solid, seeded agar medium. Then two drops of each extract was introduced into each cup aseptically, taking care to avoid spillage. This was repeated for each marked, bacteria-seeded plate of the microorganisms being used. The plates were kept for about 30 minutes to allow the extract solutions to diffuse well. The dishes were then incubated at 37°C for the 24 hours (for the bacteria) and 27°C for 48hours (for the fungi). The inhibition zone diameters (IZD) were then measured.
3.5. Determination of the MIC of Active PTLC Isolates
Four, 2-fold serial dilutions of the active PTLC Isolates (Bands 4/5 9, 17, and 19) together with the controls were obtained using DMSO. Each Petri-dish was seeded with 0.1ml of the appropriate organism. Sterile molten nutrient agar was then aseptically poured into it, shaken gently and allowed to solidify. Within each marked four-quadrants, a cup was bored using a sterile cork borer. Two drops of each dilution were placed in each cup, such that the highest concentration was opposite the lowest and never adjacent to avoid overlapping the IZDs. The inhibition zone length was calculated by subtracting the diameter of the cork borer from the measured zone of inhibition and the difference divided by 2, i.e.
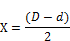
Where X= inhibition zone length
D = inhibition zone diameter
B = Diameter of cup
The minimum inhibitory concentrations (MICs) were determined by plots of logarithm of concentrations against the squares of the inhibition zone lengths. The antilogarithms of the intercept on the log conc axis give the MICs.
4. Results
These are given in the table below.
4.1. Results of Sensitivity Tests on PTLC Fractions
The results of the microbial sensitivity tests with the ethylacetate soluble PTLC fractions are shown on table 1 below.
Table 1. Results of sensitivity tests on the PTLC fractions.
| Band | Conc (mg/ml) | INHIBITION ZONE DIAMETER (MM) |
| B. subtilis | K. pneumonia | S. aureus | S. typhi | P. aeruginosa | E. coli | C. albicans | A. niger |
| 1 | 10 | 12 | 15 | 13 | 13 | 14 | 18 | - | 10 |
| 2 | 10 | 13 | 16 | 16 | 13 | 13 | 19 | 11 | 20 |
| 2/3 | 10 | 13 | 14 | 15 | 10 | 11 | 17 | - | 18 |
| 4/5 | 10 | 13 | 14 | 16 | 9 | 18 | 21 | - | 19 |
| 3 | 10 | 11 | 15 | 13 | 11 | 15 | 17 | - | 21 |
| 3x | 5 | 8 | 12 | 15 | 11 | 14 | 17 | - | 21 |
| 7/8 | 10 | 9 | 14 | 15 | 11 | 15 | 17 | - | 18 |
| 9 | 10 | 11 | 13 | 15 | 10 | 14 | 20 | 12 | 21 |
| 10 | 10 | 10 | 12 | - | 13 | - | 19 | 15 | 18 |
| 11 | 10 | 12 | 13 | - | 13 | 11 | 18 | 15 | 21 |
| 12/13 | 10 | 10 | 13 | - | 13 | - | 16 | 13 | 22 |
| 14 | 10 | 10 | 13 | - | 13 | - | 19 | 14 | 19 |
| 15 | 10 | 8 | 13 | 12 | 12 | - | 19 | 15 | 20 |
| 16 | 10 | 9 | 17 | 13 | 12 | - | 16 | 13 | 20 |
| 17 | 10 | 9 | 15 | 15 | 12 | - | 20 | 15 | 22 |
| 18* | 5 | | | | | | | | |
| 19 | 5 | 11 | 12 | - | 13 | - | 20 | 20 | 22 |
| 20 | 10 | 9 | 13 | 14 | 10 | - | 14 | 5 | 22 |
| PENG | 10 | 17 | 17 | 40 | 13 | 20 | 21 | 5 | 24 |
| CHLORAMPHENICOL | 10 | 25 | 24 | 25 | 21 | 18 | 20 | 12 | 18 |
| NYSTATIN | 5 | 16 | 16 | 13 | 16 | 16 | 19 | 40 | 35 |
*The activities of band 18 could not be determined because it was in a trace amount.
4.2. Results of the MIC Experiments
The IZDs and MICs of the various concentrations of active PTLC fractions are given in the following tables.
Table 2. Inhibition zone diameters of different concentrations of active PTLC fractions.
| Band/Micro- organism (a) K. pneumonia | Conc (mg/ml) | IZD (mm) | X | X2 | Log Conc |
| 4/5 | 5 | 10 | 2 | 4 | 0.699 |
| 2.5 | 9 | 1.5 | 2.25 | 0.398 |
| 1.25 | 9 | 1.5 | 2.25 | 0.097 |
| 0.625 | 8 | 1 | 1 | -0.204 |
| 9 | 5 | 10 | 2 | 4 | 0.699 |
| 2.5 | 9.5 | 1.75 | 3.1 | 0.398 |
| 1.25 | 9 | 1.5 | 2.25 | 0.097 |
| 0.65 | 8 | 1 | 1 | -0.204 |
| 17 | 5 | 12 | 3 | 9 | 0.699 |
| 2.5 | 10 | 2 | 4 | 0.398 |
| 1.25 | 9.5 | 1.75 | 3.1 | 0.097 |
| 0.65 | 9 | 1.5 | 2.25 | -0.204 |
| 19 | 5 | 10 | 2 | 4 | 0.699 |
| 2.5 | 9.5 | 1.75 | 3.1 | 0.398 |
| 1.25 | 9 | 1.5 | 2.25 | 0.097 |
| 0.65 | 9 | 1.5 | 2.25 | -0.204 |
| PEN. G | 5 | 13 | 3.5 | 12.25 | 0.699 |
| 2.5 | 12 | 3 | 9 | 0.398 |
| 1.25 | 12 | 3 | 9 | 0.097 |
| 0.65 | 11 | 2.5 | 6.25 | -0.204 |
| CHLORAMPHENICOL | 5 | 20 | 7 | 49 | 0.699 |
| 2.5 | 19 | 6.5 | 42.25 | 0.398 |
| 1.25 | 19 | 6.5 | 42.25 | 0.097 |
| 0.65 | 18 | 6 | 36 | -0.204 |
| (b) P. aeruginosa | | | | | |
| 4/5 | 5 | 17 | 5.5 | 30.25 | 0.699 |
| 2.5 | 15 | 4.5 | 20.25 | 0.398 |
| 1.25 | 16 | 5 | 25 | 0.097 |
| 0.625 | 12 | 3 | 9 | -0.204 |
| 9 | 5 | 15 | 4.5 | 20.25 | 0.699 |
| 2.5 | 13 | 3.5 | 12.25 | 0.398 |
| 1.25 | 12 | 3 | 9 | 0.097 |
| 0.625 | 11 | 2.5 | 6.25 | -0.204 |
| 17 | 5 | 13 | 3.5 | 12.25 | 0.699 |
| 2.5 | 12 | 3 | 9 | 0.398 |
| 1.25 | 10 | 2 | 4 | 0.097 |
| 0.625 | 13 | 3.5 | 12.25 | -0.204 |
| PEN G | 5 | 19 | 6.5 | 39.06 | 0.699 |
| 2.5 | 173.5 | 5.5 | 30.25 | 0.398 |
| 1.25 | 183 | 6 | 36 | 0.097 |
| 0.625 | 142.5 | 4 | 8 | -0.204 |
| CHLORAMPHENICOL | 5 | 193.5 | 6.5 | 39.06 | 0.699 |
| 2.5 | 173 | 5.5 | 30.25 | 0.398 |
| 1.25 | 182 | 6 | 36 | 0.097 |
| 0.625 | 143.5 | 4 | 8 | -0.204 |
| (c) E. coli. | | | | | |
| 4月5日 | 5 | 17 | 5.5 | 30.25 | 0.699 |
| 2.5 | 15.5 | 4.75 | 22.56 | 0.398 |
| 1.25 | 15 | 4.5 | 20.25 | 0.097 |
| 0.625 | 16 | 5 | 25 | -0.204 |
| 9 | 5 | 20 | 7 | 49 | 0.699 |
| 2.5 | 18 | 6 | 36 | 0.398 |
| 1.25 | 16 | 5 | 25 | 0.097 |
| 0.625 | 15 | 4.5 | 20.25 | -0.204 |
| 17 | 5 | 17 | 5.5 | 30.25 | 0.699 |
| 2.5 | 16 | 5 | 25 | 0.398 |
| 1.25 | 15 | 4.5 | 20.25 | 0.097 |
| 0.625 | 14 | 4 | 16 | -0.204 |
| 19 | 5 | 16 | 5 | 25 | 0.699 |
| 2.5 | 15 | 4.5 | 20.25 | 0.398 |
| 1.25 | 14 | 4 | 16 | 0.097 |
| 0.625 | 13 | 3.5 | 12.25 | -0.204 |
| PEN. G | 5 | 18 | 6 | 36 | 0.699 |
| 2.5 | 16.5 | 5.25 | 27.56 | 0.398 |
| 1.25 | 16 | 5 | 25 | 0.097 |
| 0.625 | 17 | 5.5 | 30.25 | -0.204 |
| CHLORAMPHENICOL | 5 | 17 | 5.5 | 30.25 | 0.699 |
| 2.5 | 15.5 | 4.75 | 22.56 | 0.398 |
| 1.25 | 15 | 4.5 | 20.25 | 0.097 |
| 0.625 | 16 | 5 | 25 | -0.204 |
The calculated MICs are shown in table 3 below
Table 3. Minimum inhibitory concentration (MICs) of active PTLC fractions.
| Fraction | Minimum inhibitory concentration (MIC) (mg/ml) |
| | E. coli | Kleb. | Ps. A |
| 4/5 | 0.36 | 0.55 | 0.59 |
| 9 | 0.32 | 0.41 | 0.40 |
| 17 | 0.35 | 0.54 | 0.60 |
| 19 | 0.60 | 0.42 | 1.38 |
| PEN. G | 0.35 | 0.40 | 0.38 |
| CHLORAMPHENICOL | 0.46 | 0.13 | 0.37 |
5. Discussion
It was surprising that none of the PTLC bands of M. myristica seed showed significant activity against B. subtilis. In a previous preliminary antimicrobial study by the researchers, the antimicrobial activity of M. myristica against B. subtilis was comparable to that of the control/pure drug (Nwaozuzu & Ebi, 2014). It is also possible that the antimicrobial activity of M. myristica against this organism resides in the ethylacetate insoluble fraction of the plant seed which showed a fairly appreciable activity against B. subtilis in another follow up study on M. myristica (Nwaozuzu & Ebi, 2016). Another study may be needed to confirm these results. Bacillus organisms are free-living, gram-positive rods that are aerobic and spore-forming. Several of the species produce potent toxins that can be lethal in experimental animals. They are ubiquitous, being found in soil, water, dust and air. They are largely seen as non-pathogenic and as contaminants when isolated in the bacteriology laboratory but occasionally they can be responsible for significant disease. Infections with bacillus organisms have also been associated with intravenous drug abuse, operative procedures, traumatic wounds, burns, hemodialysis and prosthetic heart valves and are characteristically resistant to the penicillins and cephalosporins (Poretz, 1988).
Bands 1, 2, 3, 16 and 17 were active against K. pneumonia and this is consistent with the results of the the preliminary evaluation of M. myristica crude extract in which its activity against K. pneumonia was greater than that of the control drug. The constituents of these bands may be therapeutically beneficial in infections caused by this organism like lobar pneumonia as well as those caused by other members of its family. This is of very clinical importance considering the problem associated with the treatment of klebsiella infections. K. pneumonia is a gram-negative bacilli, one of the few that causes primary lobar pneumonia, a non-motile and encapsulated organism and an important nosocomial pathogen accounting for up to 10% of hospital acquired infections (Silverblast & Weinstein, 1988). Multi-drug resistant strains have become endemic in many hospitals with the persistence of the organism associated with the continued use of large quantities of antibiotics and with the establishment of intestinal carriage among asymptomatic patients (Silverblast & Weinstein, 1988). Klebsiella is resistant to ampicillin, carbenicillin with the strain’s resistance to cephalothin, chloramphenicol, tetracycline and gentamicin increasing in frequency probably due to the acquisition of multidrug-resistant R factors. However almost all strains of the organism remain sensitive to Amikacin which has been reserved for only gentamicin-resistant organisms (Silverblast & Weinstein, 1988). The high activity of M. myristica against the klebsiella organism could make it a potential source of alternative effective antibiotics for it.
Like in the preliminary study, none of the bands had significant activity against S. aureus. S. aureus is a highly resistant gram-positive, non-spore forming bacteria with a high prevalence in both communities and hospitals. The high morbidity and mortality associated with it as well as the economic consequences and the virtual absence of a non-human reservoir makes the organism a major subject of epidemiologic studies (Waldvogel, 1988).
Only band 17 had significant activity against S. typhi indicating its potential clinical usefulness in the treatment of typhoid/enteric fever, other salmonella infections and possibly those of other gram-negative bacteria. S. typhi is the causative agent of typhoid fever (enteric fever). It is a non-spore forming gram-negative enterobacteria rod. Selection of antimicrobials for the treatment of salmonella infections has been complicated by the emergence of salmonella strains that are resistant to multiple antimicrobials (Hook, 1988). This resistance is transferred from organism to organism on plasmids that carry genetic determinants of resistance (R factors).
Against P. aeruginosa, bands 1, 4/5, 3, 3x, 7/8, and 9 were significantly active. The activity of band 4/5 was equal to that of the control indicating possible antimicrobial usefulness of the constituents of these bands in pseudomonas infections. P. aeruginosa is a gram-negative aerobic and flagellated rod belonging to the family of pseudomonadeceae. It is cosmopolitan in distribution and is sometimes present as part of the normal microbial flora of man. It rarely causes disease in normal healthy persons despite being a common human saprophyte. However, disease process as a result of infection by it begins with some alteration or circumvention of normal host defenses which may involve a disruption in the integrity of physical barriers to bacteria invasion such as the skin or mucous membranes or their circumvention as in the case of intravenous lines, urinary catheters or endotrachael tubes (Pollack, 1988). The pathogenesis of the infection from this organism is multifactorial as suggested by the large number of potential virulence factors it produces and the broad spectrum of diseases it causes. The incidence and relative frequency of hospital acquired infections from it has also been reported to be on the rise (Pollack, 1988). These hospital acquired infections as well as the other infections caused by P. aeruginosa could therefore benefit from the high antimicrobial activity of these M. myristica seed bands.
Against E. coli, all the bands showed significant antimicrobial activity except band 2. The activities of bands 9, 17, and 19 were equal to the activity of the control drug. The preliminary studies also showed the activity of crude M. myristica extract as greater than that of the control (Nwaozuzu & Ebi, 2014). The constituents of these bands may therefore be useful in E. coli infections like urinary tract infections (UTIs), bacteremia, neonatal meningitis, traveler’s diarrhea, as well as infections caused by other gram-negative aerobic enterobacteria. E. coli belongs to the family of enterobacteriacae which is a diverse group of gram-negative non-spore-forming bacilli, many of which are pathogenic to man, other animals and plants. They are aerobic but can grow under anaerobic conditions (facultative anaerobes). Many members of this group including E. coli possess plasmids which are extrachromosomal genetic elements on which genes expressing virulence properties are carried. Some of these plasmids called R factors encode for resistance to multiple antibiotics. Heavy use of antibiotics in hospitals favors the selection of R factor containing strains which might contribute to the increased antibiotic resistance of resident flora. Other R factor genes encode for the conjugal transfer of plasmids from organism to organisms even members of different species causing widespread outbreaks of nosocomial infections that have involved hundreds of patients, many institutions and several bacteria species. E. coli is the most common cause of urinary tract infections (UTIs), comprising more than 90% of infections arising outside the hospital (Sylverblatt & Weinstein, 1988). It is also the leading cause of gram-negative bacteremia in adults and treatment of these infections could benefit from the high antimicrobial activity of these M. myristica seed bands that showed significant antimicrobial activity against it.
Activity against C. albicans was generally poor with only band 19 showing a little activity though crude M. myristica seed extract gave significant activity against C. albicans. The antifungal activity of M. myristica may reside in the ethylacetate insoluble fraction M. myristica. However a replication of this study may be needed to confirm the antifungal potentials of M. myristica. C. albicans is a fungi confined to human and animal sources. They are normal commensals of man and are found on diseased skin, enteric gastrointestinal tract, expectorated sputum, the female genital tract and urine of patients with indwelling foley catheters. Interestingly it rarely colonizes normal skin but damaged skin becomes rapidly colonized with C. albicans (Edwards, 1988). Incidence of diseases due to candida has increased in frequency over the last 50 years with a relatively large number of manifestations. These may therapeutically benefit from the significant antimicrobial activity of this M. myristica seed band.
Against A. niger, bands 12/13, 17, 19 and 20 showed significant activity consistent with the significant activity of the crude extract against the organism. Fungal (mould) infections like Aspergillosis may therefore respond to treatment with agents made from constituents of these bands. A. niger is a mould that is ubiquitous in nature. It causes aspergillosis, a disease that describes an illness attributed to the antigenic stimulation, colonization or tissue invasion by aspergillus. The disease is acquired by inhalation of airborne spores (conidia) which are small enough (2.5–3.0 micrometer) to reach the alveoli or to gain entrance to paranasal sinuses with diverse clinical manifestations. Exposure to aspergillosis nearly universal but the disease is uncommon. When it occurs it could be invasive resulting in serious infections that may require surgical excision of infected body parts to contain the disease (e.g invasive aspergillosis of the brain and paranasal sinuses, non-invasive sinus colonization and possibly aspergillosis of prosthetic cardiac valve), which can aid response to chemotherapy, though prognosis in aspergillus endocarditis remains dreadful (Bennett, 1988).
Generally, the most active bands were bands 4/5, 9, 17 and 19. These bands showed significant antimicrobial activities mostly against K. pneumonia, and E. coli. The minimum inhibitory concentrations (MICs) showed that band 9 was the most potent against K. pneumonia while band 19 was most potent against P. aeruginosa. The activity of these different PTLC bands of M. myristica seed lends credence to many of its uses in Ibo-Nigeria folkloric medicine. We however recommend further in-vitro and in-vivo studies on the extracts of this plant’s seed particularly the ethylacetate insoluble fraction of the methanolic extract of the plant’s seed which the authors did not follow up in details in this serial study.
6. Conclusion
Many of the bands/constituents of the ethylacetate-soluble fraction of M. myristica seed possess significant antimicrobial activity. The most active bands were bands 4/5, 9, 17 and 19. The MIC of these bands showed that band 9 is the most potent against K. pneumonia and E. coli while band 19 is the most potent against P. aeruginosa.
Acknowledgement
Mr Paulinus Ugwu and Mr J. E Ekekwe of Botany department, University of Nigeria, Nsukka for assisting in the identification and collection of the plant materials.
Refferences
- Bennett, J.E. (1988), "Aspergillus species", in Mandell, G.L., Douglas, R.G and Benneth, J.E (Eds), Principles and practice of infectious diseases, 2nd Edition, Churchill and Livingstone, New York, Edinburgh, London, Melbourn, pp. 1447–1451.
- Edwards, J.E. (1988), "Candida species", in Mandell, G.L., Douglas, R.G and Benneth, J.E (Eds), Principles and practice of infectious diseases, 2nd Edition, Churchill and Livingstone, New York, Edinburgh, London, Melbourn, pp. 1435–1447.
- Enabulele, S.A., Oboh, F.O.J., Uwadiae., E.O. (2014). "Antimicrobial,nutritional and phytochemical properties of Monodora myristica seeds". IOSR Journal of Pharmacy and Biological Sciences, 9(4): 1-6.
- Eze-Steven, P.E., Ishiwu, C.N., Udedi, S.C., Ogeneh, B.O (2013). "Evaluation of antioxidant potential of Monodora myristica (African Nutmeg)". International Journal of Current Microbiology and Applied Sciences. 2(11):373-383.
- Hook, E.W. (1988), "Salmonella species (including typhoid fever)", in Mandell, G.L., Douglas, R.G and Benneth, J.E (Eds), Principles and practice of infectious diseases, 2nd Edition, Churchill and Livingstone, New York, Edinburgh, London, Melbourn, pp. 1256–1268.
- Nwaozuzu EE, Ebi GC (2014). "Antimicrobial screening and therapeutic potentials of crude extracts of plants used as anti-malarial remedies in Ibo-Nigeria folkloric medicine". Journal of Biology, Agriculture and Healthcare, 4 (25): 27-31.
- Nwaozuzu, E.E and Ebi, G.C. (2015), "Phytochemical analysis of the crude extract of Monodora myristica seed", International Journal of Pharmacy and Biological Sciences. 6(3): 377-382.
- Nwaozuzu EE, Ebi GC (2016). "Antimicrobial screening and therapeutic potentials of crude extracts of plants used as anti-malarial remedies in Ibo-Nigeria folkloric medicine". British Journal of Pharmaceutical Research, In Press.
- Nwozo, S.O., Kasumu, T.F., Oyinloye, B.E. (2015). "African nutmeg (Monodora myristica) lowers cholesterol and modulates lipid peroxidation in experimentally induced cholesterolemic male wistar rats". International Journal of Biomedical Sciences, 11(2): 86-92.
- Okpala, B. (2015). "Amazing benefits of Ehu seeds (Monodora myristica)". Ehu seeds, Monodora myristica.
- Owokotomo, I.A., Ekundayo, O. (2012). "Comparative study of the essential oils of Monodora myristica from Nigeria". European Chemical Bulletin, 1(7): 263-265.
- Pollack, M. (1988), "Pseudomonas aeruginosa", in Mandell, G.L., Douglas, R.G and Benneth, J.E (Eds), Principles and practice of infectious diseases, 2nd Edition, Churchill and Livingstone, New York, Edinburgh, London, Melbourn, pp. 1236–1250.
- Poretz, D.M. (1988), "Other bacillus species", in Mandell, G.L., Douglas, R.G and Benneth, J.E (Eds), Principles and practice of infectious diseases, 2nd Edition, Churchill and Livingstone, New York, Edinburgh, London, Melbourn, pp. 1184.
- Silverblatt, F.J. and Weinstein, R. (1988), "Enterobacteriaceae", in Mandell, G.L., Douglas, R.G and Benneth, J.E (Eds), Principles and practice of infectious diseases, 2nd Edition, Churchill and Livingstone, New York, Edinburgh, London, Melbourn, pp. 1226–1236.
- Tatsadjieu, L.N., Essia Ngang, J.J., Nyassoum, M.B., Etoa, F.X. (2003). "Antibacterial and antifungal activity of Xylopia aethiopica, Monodora myristica, Xanthoxylum xanthoxyloides and Xanthoxylum leprieurii from Cameroun". Fitoterapia, 74(5): 469-472.
- Waldvogel, F.A. (1988), "Staphyllococcus aureus (including toxic shock syndrome)", in Mandell, G.L., Douglas, R.G and Benneth, J.E (Eds), Principles and practice of infectious diseases, 2nd Edition, Churchill and Livingstone, New York, Edinburgh, London, Melbourn, pp. 1097–1116.

 (E. E. Nwaozuzu)
(E. E. Nwaozuzu) 



