Role of Propofol on Cardiomyocyte Under Hypoxia
Han-Fang Tseng1, Huan-Nung Chao2, Kuei-Feng Tsou1, *
1Division of Anesthesiology, Show Chwan Memoialy Hospital, Changhua, Taiwan, Republic of China
2Division of Cardiology, Show Chwan Memoialy Hospital, Changhua, Taiwan, Republic of China
Email address

(Kuei-Feng T.)
*Corresponding authors
Citation
Han-Fang Tseng, Huan-Nung Chao, Kuei-Feng Tsou. Role of Propofol on Cardiomyocyte Under Hypoxia. American Journal of Pharmacy and Pharmacology. Vol. 3, No. 4, 2016, pp. 29-32.
Abstract
Objective: Ischemic heart disease is a leading cause of death worldwide. Moreover, there is no effective therapy for preventing myocardial ischemia-reperfusion (I/R) injury. Propofol is widely used in anesthetic and has been shown to control inflammatory response, however, the role of propofol on cardiomyocyte H9c2 under hypoxia remains to be established. Method: To speculate the effect of propofol on H9c2 cells survival under hypoxia, we used new analysis tool iCELLigence system in real time and typical WST-1 assay as approaches. Results and conclusion: Results showed that propofol reversed hypoxia-induced cardiomyocyte H9c2 cells death/ growth inhibition and decreased cell viability.
Keywords
Propofol, Cardiomyocyte, Hypoxia
1. Introduction
Propofol (2, 6-diisopropylphenol) is widely used for induction and maintenance of anesthesia and for sedation in the intensive care unit [1]. The functions of propofol include anti-inflammatory, decreasing production of proinflammatory cytokines, altering expression of nitric oxide, inhibiting neutrophil function, and antioxidant properties [1,2]. Lin et al. suggested that propofol can protect against myocardial ischemia-reperfusion injury in both normal and type 2 diabetic rats, possibly by attenuating endothelial cell injury and inhibiting the apoptosis of cardiomyocytes [3]. Moreover, propofol abrogates LPS-induced TNF-α production and alleviates cardiac depression through ERK1/2 or p38 MAPK signal pathway [4]. It also has been reported that propofol ameliorates doxorubicin-induced oxidative stress and cellular apoptosis in rat isolated cardiomyocytes [5]. Taken together, propofol may serve as an adjuvant agent to protect cardiomyocyte damage under ischemia-reperfusion [6]. However, the methodology in detection of cell growth, cell proliferation, and cell apoptosis is time end point but not dynamic. Therefore, new analysis tools, called the iCELLigence System is a microelectronic biosensor system for cell-based assays, providing dynamic, real-time, label-free cellular analysis for a variety of research applications in drug development, toxicology, cancer, medical microbiology, and virology. According to previous study, the iCELLigence System has been reported to monitor endometrial cancer cell proliferation and survival by measuring cell-to-electrode responses of the cells seeded in eight-well E-plates with the integrated microelectronic sensor arrays [7]. However, the effect of propofol on cardiomyocyte under hypoxia analyzed by iCELLigence System using is uncertain. Therefore, we used new analysis tool iCELLigence system in real time and typical WST-1 assay as approaches to speculate the effect of propofol on H9c2 cells survival under hypoxia.
2. Material and Methods
2.1. Cell Lines and Cell Culture
The embryonic rat heart derived H9c2 cells were cultured in cultured at 37°C in T-25 flasks (Corning Glassworks, Corning, N. Y., USA) in DMEM (Gibco, New York, N. Y., USA) supplemented with 10% fetal bovine serum and penicillin-streptomycin (50 U/ml, Sigma, St. Louis, Mo., USA) in a 5% CO2 /95% air atmosphere. The culture medium was replaced every alternate day. Once the cells reached 70–80% confluence, they were trypsinized and seeded on 6- or 24-well plastic dishes for the following experiments.
2.2. Dunamic Monitoring of Cell Growth Using iCELLigence System
The indicated number of cells was measured by iCELLigence System according to the manufacturer’s instructions (ACEA Biosciences Inc, CA, U.S.A.). Briefly, cells were seeding into 480 μl of media in E-Plate L8 devices. The cell numbers were monitored every 15 minutes for indicated time points, depending on the experiments. The cell Index at each time point is defined as (Rn-Rb)/4.6, where Rn is the cell-electrode impedance of the well when it contains cells and Rb is the background impendence of the well with the media alone.
2.3. Cell Viability Assay
Cell viability was measured using WST-1 assay. Cells were seeded at a density of 5 x 104 cells/ml in 24-well plates and cultured in phenol red-free DMEM containing 0.5% heat-inactivated FBS for 24 h. After incubation, cells were incubated with indicated concentrations of propofol for 24 h. The WST-1 reagent was then added into the medium and incubated at 37°C for 2 h. The absorbance was measured at 450 nm in a microplate reader (Thermo Labsystems, Waltham, MA, USA).
2.4. Statistical Analysis
All experiments were performed at least three times, and the results are expressed as means 6 SEM. The statistical analyses were based on Student t test or the Mann–Whitney U test, and all calculations were performed with SigmaPlot (Systat Software, San Jose, CA). A p value, 0.05 was considered statistically significant.
3. Results
3.1. Effect of Propofol on Cardiomyocyte Under Hypoxia by iCELLigence System
To study the effect of propofol on cardiomyocyte under hypoxia, we used the iCELLigence System to monitor H9c2 cell growth in real time. Results showed that propofol attenuated hypoxia-caused growth inhibition or cell death (Fig. 1.).
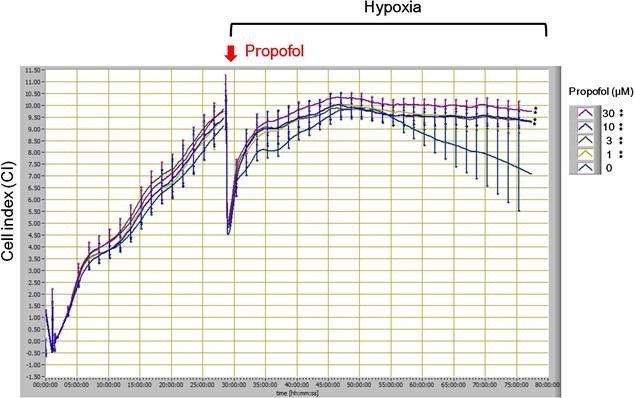
Fig. 1. Dynamic monitoring the effect propofol on H9c2 cell growth under hypoxia. All data are presented as the mean ± S. E. M. ** p <0.01 as compared with the control group (0 μM propofol, cell under hypoxia in the absence of propofol treatment).
3.2. Effect of Propofol on Cardiomyocyte Under Hypoxia by WST-1 Assay
To further confirm this phenomena, we also used the WST-1 assay to detect the cell viability under hypoxia in the absence or in the presence propofol. We observed that propofol protected hypoxia-induced cell death in a dose-dependent manner (Fig. 2.). These results suggested that propofol have an anti-cell death effect caused by hypoxia in cardiomyocyte.
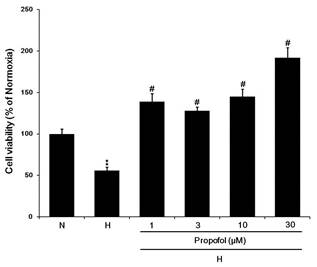
Fig. 2. Propofol reversed hypoxia-suppressed cell viability in a dose-dependent manner. N: normoxia. H: hypoxia. All data are presented as the mean ± SEM. n=3. **p < 0.01 compared to the control. #p < 0.05 compared to the corresponding data (cell under hypoxia in the absence of propofol treatment).
4. Discussion
Propofol is widely used for patients with coronary artery disease are prior to cause myocardial ischemia, which is an important factor leading to vital ventricular arrhythmias. Moreover, Liu et al. reported that propofol preconditioning suppresses ischemia-induced ventricular arrhythmias in the rat heart, which are proposed to be caused by mitochondrial KATP channels [8]. Similar results were shown in previous study, Xu et al. reported that propofol attenuates hyperglycemia-induced cardiac hypertrophy and dysfunction via heme oxygenase-1/signal transducer and activator of transcription 3 signaling pathway in streptozotocin-induced type 1 diabetes rats [9]. Sun et al. also suggested that propofol protected doxorubicin-induced toxicity in rat cardiomyocytes via PI3K-Akt-Bad signaling pathway and associated with ROS production [10]. Moreover, it is also have been reported that propofol protects the immature rabbit heart against ischemia and reperfusion injury. Shirakawa et al. reported that the efficacy of propofol at 2 μg/mL was similar effect of cyclosporine A on cardiomyocyte protection. They also concluded that propofol at a clinical concentration is cardioprotective in the immature heart [11]. Tang et al. suggested that propofol significantly improved myocardial depression and increased survival rate of mice after lipopolysaccharide (LPS) treatment and are associated with reduced myocardial TNF-α production, gp91phox, ERK1/2, and p38 MAPK [4]. In our knowledge, the progress in therapeutic drugs and devices to treat heart failure (HF) caused by ischemia during the last few years, the clinical outcome of this disease remains deleterious. Impaired left ventricular function leads to the imbalance of the redox homeostasis toward increased concentrations of reactive oxygen species (ROS) and causes inflammatory processes within the vasculature in heart failure [12]. Interestingly, it is well known that neutrophils play an importantly role in acute and chronic inflammatory processes, including myocardial ischemia/reperfusion injury [13]. Furthermore, Yang et al. also reported that propofol significantly reduced superoxide generation, elastase release, and chemotaxis in human neutrophils activated by fMLF [13]. Therefore, we hypotheses propofol may have therapeutic or preventive potential to attenuate hypoxia or ischemia causes cardiomyocyte cell death via neutrophils-ROS-inflammatory cascade, however, we need more evidences to prove it.
Interestingly, the whole plant methanolic extract of Enicostemma littorale protect to ROS cause Islets of Langerhans to apoptosis [14]. Moreover, Kuo et al. reported that induction of apoptosis in HSC-T6 by oridonin, which is a diterpenoid extracted from Rabdosia rubescens is associated with a decrease in cellular GSH level and increase in ROS production [15]. Oridonin has also been reported that has the potential to be developed as an anticancer agent, and the combination of oridonin with those agents leading to reduction of caspase-9 expression in the human laryngeal cancer HEp-2 cells could represent a novel approach to human laryngeal cancer treatment [16]. Similar results were shown tht β-Elemene is a natural anticancer compound extracted from the Chinese medicinal herb Curcuma Wenyujin. β-elemene is proapoptotic effective in rheumatoid arthritis fibroblast-like synoviocytes (RA-FLS), which is mediated through induction of ROS formation via mitochondria and p38 MAPK activation. Authors suggested that β-Elemene may thus have therapeutic benefits for rheumatoid arthritis [17]. Methylophiopogonanone A (MO-A), an active homoisoflavonoid of the Chinese herb Ophiopogon japonicus which has been shown to have protective effects on cerebral ischemia/reperfusion (I/R) injury via ROS and MMP-9 associated pathways [18]. Resveratrol, a natural polyphenolic phytochemical, has been reported that it has growth-inhibitory effect on HepG2 cells via caspase-9 and caspase-3 pathways. After combonation of matrine, a natural component extracted from the traditional Chinese medical herb Sophora flavescens Ait, results showed that it ehanced the resveratrol, growth-inhibitory effect of resveratrol on HepG2 cells, which could be assocaited with activation of caspase-3 and caspase-9, downregulation of survivin, induction of reactive oxygen species (ROS) generation and disruption of mitochondria membrane potential (Δψm) [19]. Importantly, it also has been reported that the Nardostachyos Radix et Rhizoma (NRR) is a well-known medicinal herb widely used in Chinese, Uyghur and Ayurvedic medicines for the treatment of cardiovascular diseases. Additionally, NRR volatile oil prevented the oxidative stress-induced H9c2 cell death in cells via reducing intracellular ROS production, inducing antioxidant enzymes and activating Akt phosphorylation [20]. Salvianolic acid B (SalB), the main water-soluble bioactive compounds isolated from the traditional Chinese medical herb Danshen, has been reported that induces apoptosis in human glioma U87 cells via p38-mediated ROS generation [21]. Taken together, we suggest that the traditional Chinese medicine extract or natural compouds may represent a novel approach to human disease in the future.
References
- Marik, P. E. Propofol: an immunomodulating agent. Pharmacotherapy 25:28S-33S; 2005.
- Allaouchiche, B.; Debon, R.; Goudable, J.; Chassard, D.; Duflo, F. Oxidative stress status during exposure to propofol, sevoflurane and desflurane. Anesthesia and analgesia 93:981-985; 2001.
- Lin, C.; Sui, H.; Gu, J.; Yang, X.; Deng, L.; Li, W.; Ding, W.; Li, D.; Yang, Y. Effect and mechanism of propofol on myocardial ischemia reperfusion injury in type 2 diabetic rats. Microvascular research 90:162-168; 2013.
- Tang, J.; Hu, J. J.; Lu, C. H.; Liang, J. N.; Xiao, J. F.; Liu, Y. T.; Lin, C. S.; Qin, Z. S. Propofol inhibits lipopolysaccharide-induced tumor necrosis factor-alpha expression and myocardial depression through decreasing the generation of superoxide anion in cardiomyocytes. Oxidative medicine and cellular longevity 2014:157376; 2014.
- Lai, H. C.; Yeh, Y. C.; Wang, L. C.; Ting, C. T.; Lee, W. L.; Lee, H. W.; Wang, K. Y.; Wu, A.; Su, C. S.; Liu, T. J. Propofol ameliorates doxorubicin-induced oxidative stress and cellular apoptosis in rat cardiomyocytes. Toxicology and applied pharmacology 257:437-448; 2011.
- King, N.; Al Shaama, M.; Suleiman, M. S. Propofol improves recovery of the isolated working hypertrophic heart from ischaemia-reperfusion. Pflugers Archiv: European journal of physiology 464:513-522; 2012.
- Koval, O. A.; Sakaeva, G. R.; Fomin, A. S.; Nushtaeva, A. A.; Semenov, D. V.; Kuligina, E. V.; Gulyaeva, L. F.; Gerasimov, A. V.; Richter, V. A. Sensitivity of endometrial cancer cells from primary human tumor samples to new potential anticancer peptide lactaptin. Journal of cancer research and therapeutics 11:345-351; 2015.
- Liu, Q.; Yao, J. Y.; Qian, C.; Chen, R.; Li, X. Y.; Liu, S. W.; Sun, B. G.; Song, L. S.; Hong, J. Effects of propofol on ischemia-induced ventricular arrhythmias and mitochondrial ATP-sensitive potassium channels. Acta pharmacologica Sinica 33:1495-1501; 2012.
- Xu, J.; Li, H.; Irwin, M. G.; Xia, Z. Y.; Mao, X.; Lei, S.; Wong, G. T.; Hung, V.; Cheung, C. W.; Fang, X.; Clanachan, A. S.; Xia, Z. Propofol ameliorates hyperglycemia-induced cardiac hypertrophy and dysfunction via heme oxygenase-1/signal transducer and activator of transcription 3 signaling pathway in rats. Critical care medicine 42:e583-594; 2014.
- Sun, X.; Gu, J.; Chi, M.; Li, M.; Lei, S.; Wang, G. Activation of PI3K-Akt through taurine is critical for propofol to protect rat cardiomyocytes from doxorubicin-induced toxicity. Canadian journal of physiology and pharmacology 92:155-161; 2014.
- Shirakawa, M.; Imura, H.; Nitta, T. Propofol protects the immature rabbit heart against ischemia and reperfusion injury: impact on functional recovery and histopathological changes. BioMed research international 2014:601250; 2014.
- Konradi, J.; Mollenhauer, M.; Baldus, S.; Klinke, A. Redox-sensitive mechanisms underlying vascular dysfunction in heart failure. Free radical research 49:721-742; 2015.
- Yang, S. C.; Chung, P. J.; Ho, C. M.; Kuo, C. Y.; Hung, M. F.; Huang, Y. T.; Chang, W. Y.; Chang, Y. W.; Chan, K. H.; Hwang, T. L. Propofol inhibits superoxide production, elastase release, and chemotaxis in formyl peptide-activated human neutrophils by blocking formyl peptide receptor 1. Journal of immunology 190:6511-6519; 2013.
- Srivastava, A.; Bhatt, N. M.; Patel, T. P.; Dadheech, N.; Singh, A.; Gupta, S. Anti-apoptotic and cytoprotective effect of Enicostemma littorale against oxidative stress in Islets of Langerhans. Pharmaceutical biology:1-12; 2016.
- Kuo, L. M.; Kuo, C. Y.; Lin, C. Y.; Hung, M. F.; Shen, J. J.; Hwang, T. L. Intracellular glutathione depletion by oridonin leads to apoptosis in hepatic stellate cells. Molecules 19:3327-3344; 2014.
- Kang, N.; Cao, S. J.; Zhou, Y.; He, H.; Tashiro, S.; Onodera, S.; Qiu, F.; Ikejima, T. Inhibition of caspase-9 by oridonin, a diterpenoid isolated from Rabdosia rubescens, augments apoptosis in human laryngeal cancer cells. International journal of oncology 47:2045-2056; 2015.
- Zou, S.; Wang, C.; Cui, Z.; Guo, P.; Meng, Q.; Shi, X.; Gao, Y.; Yang, G.; Han, Z. beta-Elemene induces apoptosis of human rheumatoid arthritis fibroblast-like synoviocytes via reactive oxygen species-dependent activation of p38 mitogen-activated protein kinase. Pharmacological reports: PR 68:7-11; 2016.
- Lin, M.; Sun, W.; Gong, W.; Zhou, Z.; Ding, Y.; Hou, Q. Methylophiopogonanone A Protects against Cerebral Ischemia/Reperfusion Injury and Attenuates Blood-Brain Barrier Disruption In Vitro. PloS one 10:e0124558; 2015.
- Ou, X.; Chen, Y.; Cheng, X.; Zhang, X.; He, Q. Potentiation of resveratrol-induced apoptosis by matrine in human hepatoma HepG2 cells. Oncology reports 32:2803-2809; 2014.
- Maiwulanjiang, M.; Chen, J.; Xin, G.; Gong, A. G.; Miernisha, A.; Du, C. Y.; Lau, K. M.; Lee, P. S.; Chen, J.; Dong, T. T.; Aisa, H. A.; Tsim, K. W. The volatile oil of Nardostachyos Radix et Rhizoma inhibits the oxidative stress-induced cell injury via reactive oxygen species scavenging and Akt activation in H9c2 cardiomyocyte. Journal of ethnopharmacology 153:491-498; 2014.
- Wang, Z. S.; Luo, P.; Dai, S. H.; Liu, Z. B.; Zheng, X. R.; Chen, T. Salvianolic acid B induces apoptosis in human glioma U87 cells through p38-mediated ROS generation. Cellular and molecular neurobiology 33:921-928; 2013.

 (Kuei-Feng T.)
(Kuei-Feng T.) 



