| 1. | ||
| 2. | ||
| 2.1. | ||
| 2.2. | ||
| 3. | ||
| 3.1. | ||
| 3.2. | ||
| 4. | ||
Pharmaceutical Quality Assurance of Omeprazole Capsule Brands Commonly Used in Health Institutions in Southern Nigeria
Okorie O., Azaka J. E., Amadi C. M.
Department of Pharmaceutics and Pharmaceutical Technology, Faculty of Pharmaceutical Sciences, University of Port Harcourt, Rivers State, Nigeria
Email address
 (Azaka J. E.)
(Azaka J. E.) Citation
Okorie O., Azaka J. E., Amadi C. M. Pharmaceutical Quality Assurance of Omeprazole Capsule Brands Commonly Used in Health Institutions in Southern Nigeria. American Journal of Pharmacy and Pharmacology. Vol. 3, No. 4, 2016, pp. 33-39.
Abstract
Generic substitution and cost of different brands of drugs has been a major challenge between health care professionals and their patients especially in health institutions. There is a need to enlighten prescribers on the quality of generic drugs used in the hospitals so as to ensure that efficacious, cost-effective drugs are prescribed for the patients and hence, quality of life is improved. The proliferation of counterfeit and poor-quality drugs is a major public health problem in developing countries such as Nigeria. The objective of this study was to compare the Pharmaceutical quality of innovator brand of Omeprazole capsule (Losec®) with its generic brands that are commonly prescribed in Southern Nigeria. Ten generic brands of Omeprazole capsule were assessed relative to Losec®. Physicochemical tests were performed on all brand samples using standard methods. Weight uniformity, content of active ingredient and disintegration tests were performed on all the samples using Pharmacopoeial and other standard methods. The drug release profiles were evaluated in-vitro using a dissolution test apparatus. Of the 11 brands that were tested, (n=11, 100%) met the specifications for physicochemical properties, disintegration tests and drug release, (n=10, 90.9%) met the specification for weight uniformity while (n=9, 81.8%) brands complied with the specification for drug content. None of the samples were suspected to be counterfeit, based on visual inspection and assessment. Statistical evaluation of the results revealed remarkable inter-brand variations and significant differences between the generic brands and the innovator brand. This clearly raises a question about the interchangeability between the innovator brand and its generic counterparts and even among the generics themselves. It also highlights the need for proper assessment and monitoring of patients after performing generic substitution.
Keywords
Omeprazole Capsules, Quality Assurance, Generic Substitution, Inter-brand Variation, Public Health
1. Introduction
Omeprazole, a selective and irreversible proton pump inhibitor which has greater anti-secretory activity than H2 antagonists has been widely used for the treatment of Gastroesophageal reflux disease (GERD), Erosive esophagitis, Zollinger-Ellison syndrome and Peptic ulcer disease [1, 2]. It suppresses stomach acid secretion by specific inhibition of the H+/K+ ATPase system found at the secretory surface of gastric parietal cells [3]. Due to its degradation under acidic pH, omeprazole is formulated as enteric coated pellets encased in hard gelatin capsules [4]. Pellets are capable of undergoing changes upon storage, involving mainly enteric performance and release characteristics [5]. Additional storage-induced changes in the capsule shell may take place after encasing into capsules [6, 7]. Hence, post-marketing follow-up is essential to monitor probable changes which may affect the performance of omeprazole capsules [8].
Omeprazole is currently marketed in Nigeria by a number of pharmaceutical companies using coated micro-granules and packaging materials from Asia [9]. However, there is a likelihood of variations in the pharmaceutical quality of generic brands of omeprazole which can be attributed to several formulation factors. For example; differences in the quality of granule coating may be a source of variability in the in-vitro and in-vivo availability of omeprazole [1]. Packaging types have also been reported to be an additional factor significantly influencing formulae stability and performance [10].
Wide variations in prices of marketed omeprazole capsules currently exists, particularly between generic products and the proprietary one (Innovator brand). A generic drug formulation contains the same active pharmaceutical ingredient(s) in the same quantity as the reference listed drug (RLD) or innovator brand. A generic medicine is defined as an exact simulation of an established drug, not protected by a patent and promoted with the chemical name of the active ingredient. Generic formulations of branded drugs offer therapeutic alternatives for physicians and the patients they treat. However, there are legitimate concerns about the efficacy of generic formulations and data show that cost-savings to the patient or the health care system may not be as significant as expected [11]. Most Physicians, based on acquired experience, personal preference or an interpretation of specific data, identify certain branded products that they typically prescribe to be "dispensed" as written" (DAW). While regulations vary somewhat from country to country, writing "DAW" or "brand required" on a prescription typically means that the pharmacy must provide the branded products indicated. When no such indication is present, the pharmacist dispenses a generic, if available. Due to a significantly higher profit margin associated with most generics compared to branded formulations, the retailers may also pressure the Pharmacist to substitute [12].
Private health institutions have been found to charge up to 184% more than the public health facilities and 193% more than private retail pharmacies. The Innovator brand of omeprazole was found to cost between 2-7 times the lowest priced generic equivalents. Despite the fact that generic medicines are more available than innovator brands in public and private health clinics and the similarity in chemical structure, mechanism of action and effectiveness amongst Proton pump inhibitors (PPIs), health plans and strategies are implemented in some health institutions to favor the use of omeprazole over other PPIs [6]. This act increased the physicians’ generic prescribing ratio and also promotes therapeutic substitution from brand name PPI to generically available omeprazole while maintaining similar clinical effectiveness [13].
The proliferation of counterfeit and poor-quality drugs is a major public health problem; especially in developing countries lacking adequate resources to effectively monitor their prevalence. Currently, there are no reliable statistics on the level of incidence of fake drugs in Nigeria [14]. From previous studies, estimates of the extent of counterfeit medicines in circulation in Nigeria ranged from 25% to 80% [15-19]. Counterfeiting can apply to both branded and generic products and counterfeit products may include products with the correct ingredients or with the wrong ingredients, without active ingredients, with insufficient active ingredients or with fake packaging. From a public health point of view, counterfeit/substandard drugs have eroded the confidence of the public on the healthcare delivery system. The adverse effects may include treatment failures, organ dysfunction/damage, worsening of chronic disease conditions and death [15]. When medicines containing little or no active ingredients whether counterfeit or substandard are used for the treatment of common ailments with a high untreated mortality, morbidity and mortality will likely be on the increase. There is also the problem of financial losses for the pharmaceutical industry [13].
This study is carried out to assess the pharmaceutical quality of the generic brands of omeprazole capsule commonly used in health institutions in Southern Nigeria relative to the Proprietary product (Losec®). It also attempts to highlight the incidence of counterfeiting (if any) of omeprazole capsules on sale in Southern Nigeria.
2. Materials and Method
2.1. Materials
Omeprazole standard pure sample and the innovator brand (Losec®) were obtained from Astra Zeneca Pharmaceuticals LTD, Sweden while the generic brands (Meprasil®, Omfil®, Zim®, Sicovid®, Strozole®, Glack®, Prasol®, Acinil®, Omecet®, Omecap®) of omeprazole capsule were purchased from approved pharmacies in Southern Nigeria. These samples were stored at temperature <25°C, in a cool place, prevented from having direct access to light and thereafter, tested within their expiration dates. Table 1 provides a brief description of the Omeprazole brands sampled. Others include; 0.1N Hydrochloric acid, 0.1N Sodium hydroxide solution, Ethanol, Distilled water, Phosphate buffer pH 6.8, Distilled water, Filter papers (Whatman), Glass test tubes (Pyrex), Thermometer, Dissolution tester (Erweka, DT-600, Germany), Disintegration apparatus (Erweka, ZT 122, Germany), UV/Vis Spectrophotometer (Jenway 6405), pH meter (PHS-25 pH meter, China), Electro thermal melting point apparatus (Frost instruments limited, USA).
2.2. Methods
2.2.1. Sample Collection
Drug quotations and stock of omeprazole capsules were obtained from the pharmacy department of health institutions representing the south east, south west and south-south so as to ascertain the specific brands of omeprazole capsules used.
2.2.2. Packaging and Labelling Inspection
The primary and secondary packages of the different brands were examined carefully to check for required information such as product name, manufacturers address, manufacturing dates, batch numbers, expiry date, amount of active ingredients and National Agency for Food and Drug Administration and Control (NAFDAC) Registration number.
2.2.3. Physicochemical Analysis
(i). Test for organoleptic property
The Organoleptic properties of the different brands e.g. colour of capsule shell, colour of the drug before and after exposure to light, odour of the drug and texture of the drug were determined by inspection. These properties are directly related to the chemistry of the drug and as such can serve as a non-specific identification test of the drug [20].
(ii). Solubility test
The drug content in the capsules of each brand was emptied into a porcelain mortar, crushed and 250mg each of the resulting powder was weighed and dissolved in 3 beakers containing 25ml of distilled water, 25ml of 96% ethanol (prepared by diluting 24ml of absolute ethanol up to 25ml using distilled water), 10% sodium hydroxide solution (prepared by dissolving 2.5g of sodium hydroxide pellets in 25ml of distilled water) respectively. The three beakers were stirred using a glass stirrer for 5minutes each and allowed to stand for 15 minutes. This test was carried out for all the brands and the results obtained were recorded. The British Pharmacopoeia [20] specifies that omeprazole is very slightly soluble in water, sparingly soluble in 96% ethanol and readily dissolves in dilute solutions of alkali hydroxides.
(iii). pH Determination
The pH meter was calibrated with standard buffer solutions of pH 4.0, 7.0 and 9.10. The drug content in the capsules of each brand was emptied into a porcelain mortar, crushed and 250mg of the resulting powder was weighed and dissolved in 25ml of distilled water. The pH was measured by inserting the electrode of the pH meter into the drug solution and the reading was taken after stabilization. This was done in duplicates and the procedure repeated for each brand [6].
(iv). Melting point Determination
Powdered drug from each brand were introduced into different capillary tubes and then inserted into the melting point apparatus. The melting points were observed and recorded for the different samples.
2.2.4. Quality Assurance Tests
(i). Weight uniformity
The BP and USP methods were used to evaluate the omeprazole capsules. Weight uniformity was evaluated by individually weighing the capsules from each brand and recording their respective weights. The content of the capsule were removed without altering the integrity of the capsule shell, and the weight of the individual empty capsule shell was determined. The net weight of each capsule content was determined by subtracting the weight of each empty capsule shell from the initial capsule weight. This test was carried out for all the brands and the values obtained were recorded. The mean weight, standard deviation and percentage standard deviation were determined [20, 21].
(ii). Uniformity of content
The content of active ingredient of each brand sample was determined using an Ultraviolet (UV) spectrophotometer (Jenway, 6405), according to the method described by D. Kumaraswami et al. [22].
(iii). Disintegration test
The disintegration time for all the omeprazole capsule brands was determined according to the USP method [21] using a disintegration apparatus (Erweka, ZT 122, Germany). 1000ml of 0.1N Phosphate buffer (pH 6.8) maintained at 37 ± 1°C was used as the immersion fluid.
(iv). Dissolution test
The release rate of omeprazole from the capsules was conducted with the basket method in a dissolution apparatus (Erweka DT-600, Germany) in 900ml of phosphate buffer (pH 6.8) maintained at 37 ± 1°C. The basket speed was set at 100rpm and the procedure lasted for 60 min. Aliquot portions (5ml) samples were withdrawn at intervals of 10 min and replaced with the same volume of phosphate buffer. These samples were assayed for drug content using UV spectrophotometric method of analysis described by D. Kumaraswami et al. [22].
(v). Data analysis and statistics
Inter-brand variation was evaluated using the one-way analysis of variance (ANOVA) followed by Dunnet multiple comparison post-test to compare each of the local brands with the innovator. P≤0.05 was considered statistically significant.
Table 1. Description of the Omeprazole capsule brands tested.
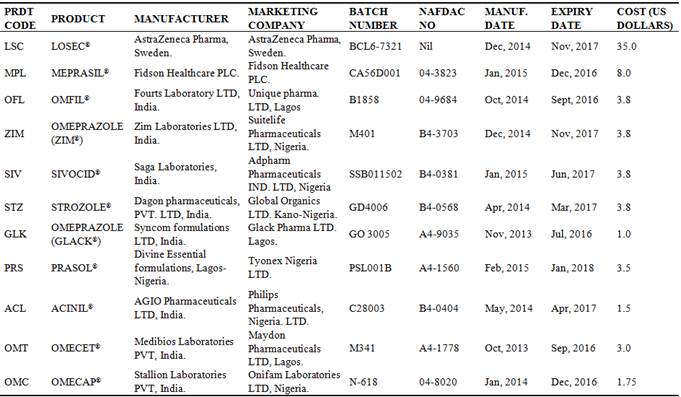
**1 US Dollars is equivalent to 199.99naira
3. Results and Discussion
3.1. Physical Assessment: Physicochemical Analysis
On inspection of the packs and labels on the primary and secondary containers of the different brands used for the analysis, all brands met the BP [20] specification for packaging and labelling of omeprazole capsules. The results obtained for the physicochemical analysis and solubility are presented in Table 2. These results show that the physicochemical properties of the tested brands of omeprazole capsules conformed to the USP and BP specifications [20, 21]. The pH of a pharmaceutical product is a measure of its acidity/alkalinity. It is a very critical factor in the formulation of pharmaceutical products as it influences the solubility, stability and palatability of the product. The product pH may reflect the intrinsic pH of the active pharmaceutical ingredient. The melting point of a drug gives an indication of its purity. A pure substance is characterized by a very sharp melting point. Altered values of melting point temperature may indicate an admixed, impure substance or degradation product(s) [23]. It is also significant as a specific method/test for identification. All the brands maintained an alkaline pH; this justifies their formulation as gastro-resistant capsules and also gives an idea of their stability profile. The melting point of the generic brands ranged from 156-170°C, corresponding to 100-112% of that of the innovator brand. This variation in melting point between the different brands sampled may have resulted from differences in the excipients used in the formulation as the degree to which melting point is lowered is dependent on the excipients used in the formulation [24].
Table 2. Physical description, physicochemical properties and solubility of innovator and generic brands.
| PRODUCT CODE | PHYSICAL DESCRIPTION | PHYSICOCHEMICAL CHARACTERISTICS | SOLUBILITY | |||||
| Appearance | Colour | Smell | pH | Melting point | Solubilty (Water) | Solubilty (96% Ethanol) | Solubilty (0.1n NaOH) | |
| LSC | Pellets | White | Foul smell | 7.20 | 151-155 | Slightly soluble | Sparingly soluble | Very soluble |
| MPL | Pellets | White | Foul smell | 6.69 | 158-162 | Slightly soluble | Sparingly soluble | Very soluble |
| OFL | Pellets | White | Foul smell | 6.82 | 160-165 | Slightly soluble | Sparingly soluble | Very soluble |
| ZIM | Pellets | White | Foul smell | 7.01 | 162-170 | Slightly soluble | Sparingly soluble | Very soluble |
| SIV | Pellets | White | Foul smell | 6.95 | 162-168 | Slightly soluble | Sparingly soluble | Very soluble |
| STZ | Pellets | White | Foul smell | 6.75 | 160-168 | Slightly soluble | Sparingly soluble | Very soluble |
| GLK | Pellets | White | Foul smell | 7.29 | 161-168 | Slightly soluble | Sparingly soluble | Very soluble |
| PRS | Pellets | White | Foul smell | 6.65 | 158-163 | Slightly soluble | Sparingly soluble | Very soluble |
| ACL | Pellets | White | Foul smell | 7.30 | 156-160 | Slightly soluble | Sparingly soluble | Very soluble |
| OMT | Pellets | White | Foul smell | 6.91 | 160-166 | Slightly soluble | Sparingly soluble | Very soluble |
| OMC | Pellets | White | Foul smell | 6.86 | 158-162 | Slightly soluble | Sparingly soluble | Very soluble |
3.2. Pharmaceutical Quality Analysis
The average weight of all brand samples tested except PRS where within USP [21] specification for uniformity of weight of single dose preparations. They fell within 92.68-103.8% of the innovator product. This implies that there is an acceptable variation from the innovator brand. Although the intra-batch variation was high in two brands: PRS and GLK. The implication of this is that both brands may produce variable plasma concentration in the patient. ANOVA was adopted to compare the variation of the average weight of all brand samples tested. Results of ANOVA showed that there was significant influence of brand on the average weight of drug contained in each capsule (P<0.05). The variations in the average weights of the different brands tested are attributed to formulation and manufacturing difference as well as poor product handling.
The drug content of all the brands met the USP [21] specification as they all fell within 90-110% of the label claim except PRS and OMT which has drug content of 114.33% and 83.52% respectively. The drug content of all the brands tested were uniform and were compliant to the official compendia because all the individual capsule assayed had a drug content range of 85%-115% and none of them had a relative standard deviation value (RSD) above 6.0. The high drug content of PRS can be attributed to its variation in weight. This can significantly affect dosage uniformity and indicates possible formulation errors and non-compliance to current Good Manufacturing Practice.
Table 3. Average weight and % drug content of innovator and generic brands.
| PRODUCT CODE | AVERAGE WEIGHT | % DRUG CONTENT | |||||
| Average Weight (Mg) Sem | % Deviation | Remark | Average Absorbance | Conc (µg/ml) | Label Claim | % Content | |
| LSC | 290.6 | 0.93 | PASSED | 0.151 | 6.05 | 20mg | 100.99 |
| MPL | 287.5 | 4.29 | PASSED | 0.153 | 6.12 | 20mg | 102.00 |
| OFL | 301.7 | 2.32 | PASSED | 0.155 | 6.22 | 20mg | 103.68 |
| ZIM | 297.5 | 2.06 | PASSED | 0.143 | 5.72 | 20mg | 95.33 |
| SIV | 274.1 | 2.37 | PASSED | 0.149 | 5.97 | 20mg | 99.52 |
| STZ | 292.6 | 3.24 | PASSED | 0.151 | 6.03 | 20mg | 100.99 |
| GLK | 263.0 | 2.13 | PASSED | 0.143 | 5.72 | 20mg | 95.33 |
| PRS | 279.8 | 7.74 | FAILED | 0.171 | 7.40 | 20mg | 114.33 |
| ACL | 280.2 | 2.65 | PASSED | 0.153 | 6.12 | 20mg | 102.08 |
| OMT | 271.5 | 1.67 | PASSED | 0.125 | 5.01 | 20mg | 83.52 |
| OMC | 281.3 | 1.87 | PASSED | 0.139 | 5.57 | 20mg | 92.83 |
Table 4. Disintegration time and release rate of innovator and generic brands.
| PRODUCT CODE | DISINTEGRATION | DISSOLUTION (RELEASE RATE) | ||
| Disintegration time (min) | F1 | F2 | %DE | |
| LSC | 2.05 | Reference | Reference | 93.95 |
| MPL | 2.26 | 8.75 | 65.40 | 80.97 |
| OFL | 2.17 | 5.58 | 68.94 | 91.44 |
| ZIM | 2.05 | 12.03 | 61.45 | 87.00 |
| SIV | 1.77 | 11.80 | 63.73 | 90.10 |
| STZ | 2.24 | 12.40 | 66.19 | 91.00 |
| GLK | 2.29 | 14.18 | 54.57 | 78.85 |
| PRS | 2.44 | 16.53 | 50.32 | 95.60 |
| ACL | 2.18 | 19.21 | 48.65 | 79.55 |
| OMT | 2.31 | 30.71 | 41.48 | 93.90 |
| OMC | 1.58 | 27.60 | 43.61 | 85.50 |
F1=Differential factor, F2=Similarity factor, %DE=Percentage dissolution efficiency
The result of the disintegration and dissolution tests are presented in Table 4. The capsule shells of all the brands sampled readily released their content at a pH of 6.8. The average disintegration time of all brand samples tested where within USP [21] specification for disintegration of gastro-resistant capsule preparations. They fall within 77-119% of the innovator product implying an acceptable variation from the innovator brand. However, the variations in disintegration time of the different brands tested can be attributed to capsule shell formulation and manufacturing difference. Results of ANOVA showed that there was significant influence of brand on the disintegration time of the capsules (p-value<0.05).
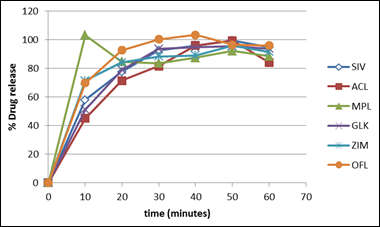
Fig. 1. Dissolution profile of some brands of omeprazole capsules.
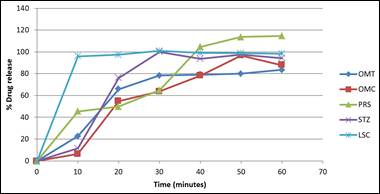
Fig. 2. Dissolution profile of some brands of omeprazole capsules.
Dissolution is the amount of substance that goes into solution per unit time under standardised conditions of liquid/solid interface, solvent composition and temperature. It is one of the most important tools to predict the in-vivo bioavailability and in some cases to determine bioequivalence and assure interchangeability [25]. Dissolution testing involves dissolving the solid dosage form of a drug compound under controlled conditions, followed by collection and analysis of the sample to determine the percentage of drug dissolved at certain time point. The results of this study were expressed as % (95% Confidence Intervals (CI)). Variations were evaluated using the one-way analysis of variance (ANOVA) and P≤0.05 was considered statistically significant. Dissolution profile compares the percentage of a drug substances dissolved relating to time and represents an alternative to assessment of solid forms before clinical tests [26].
All the brands tested except PRS and OMC met the specification for dissolution as specified in the USP [21] because 75% of their drug content was released within the first 30 minutes using phosphate buffer pH 6.8 as dissolution medium while that of PRS and OMC were 64.73% and 63.73 respectively. From the dissolution profile plot, MPL released all of its content before 10minutes implying that the brand will likely have a rapid onset of action. At 10 minutes, all the brands had above 20% drug release except OMC and STZ. This can be attributed to the enteric coating of the capsule shell and can be confirmed from the disintegration test of STZ which revealed a higher disintegration time than others in the same medium. None of the brands released their content during the acidic phase of dissolution testing. This is because the capsule shell readily protects the encapsulated omeprazole from degradation and instability due to the low pH.
The dissolution profile of the innovator brand was compared with that of the other generic brands with respect to the F1, F2 and percentage dissolution efficiency values which were calculated. On comparison, all the tested brands can be said to be bioequivalent to the innovator brand because their F1 values were within the range of 0-15 while their F2 values were between 50-100 [27] except PRS, ACL, OMC, and OMT whose F1 and F2 values were above 15 and below 50 respectively. Statistical evaluation of the release data indicated significant inter-brand variation in the first 10 minutes of the release test, as well as significant differences between the innovator and each of the generic brands. In addition, the innovator showed statistically significant differences regarding the extent of omeprazole release (%released at 45 minutes) when compared to other brands. Results of ANOVA showed that there was significant influence of brand on the drug release rate of capsules (P≤0.05). The variations in the percentage drug release of the different brands tested can be attributed to manufacturing difference.
4. Conclusion
The Quality assurance tests carried out on the innovator and ten generic brands of omeprazole capsules commonly used in health institutions in Southern Nigeria using standard quality control tests of weight uniformity, uniformity of content, disintegration tests and dissolution tests with the aim of evaluating whether they are pharmaceutically and/or therapeutically equivalent revealed remarkable inter-brand variations and significant differences between the generic brands and the innovator brand.The results clearly raise a question about the interchangeability between the innovator brand and its generic counterparts and even among the generics themselves. The authors therefore suggest the need for proper assessment and monitoring of patients after performing generic substitution.Also, appropriate regulation regarding drug substitution should be strengthened to improve the quality of health care delivery. Healthcare providers should take into account that although there are obvious economic benefits (less cost) of using generic medicines, there is a clear need to monitor patients upon generic substitution especially for certain drugs such as chemotherapeutic drugs and drugs with narrow therapeutic index. The importance of continuous quality control and post-marketing analysis of drugs by regulatory agencies and researchers in both developing and developed countries cannot be over-emphasized.
Competing Interests
The authors declare that they have no competing interests.
References