Preparation of Novel Antimicrobial Meat Packaging Using Chitosan-Arginine
R. A. Lahmera1, *, A. P. Williamsb2, S. Townsendc3, S. Bakerc3,
D. L. Jonesb2
1Department of Food Science and Technology, Faculty of Agriculture, University of Tripoli. Tripoli, Libya
2School of Environment, Natural Resources & Geography, College of Natural Sciences, Bangor University, Bangor, LL57 2UW, UK
3Synedgen, Inc., 1420 N Claremont Blvd Suite 105D, Claremont, CA 91711, USA
Email address

(R. A. Lahmer)
*Corresponding author
Citation
R. A. Lahmera, A. P. Williamsb, S. Townsendc, S. Bakerc, D. L. Jonesb. Preparation of Novel Antimicrobial Meat Packaging Using Chitosan-Arginine. American Journal of Food, Nutrition and Health. Vol. 1, No. 2, 2016, pp. 7-12.
Abstract
Chitosan-arginine (Ch-arg) has been proposed as an anti-microbial agent to reduce the proliferation of spoilage and pathogenic bacteria within meat products destined for human consumption. In the current experiment its use as an antimicrobial packaging material was examined. Two different concentrations of chitosan-arginine (0.05 and 0.15% w/w) were blended into a cellulose film (Ch-arg film). When placed in contact with chicken and beef juice inoculated with a lux-marked strain of E. coli O157, the film incorporating the highest Ch-arg concentration resulted in a small reduction of E. coli O157 in chicken juice; however, there was no effect of the Ch-arg film on E. coli O157 in beef juice. The lack of observed effect in the beef juice experiment we ascribe to insufficient surface-to-surface contact between the film and the bacteria in the beef juice and the greater presence of other Ch-arg reactive components in the juice (e.g. fats, blood cells). Results suggest that, in combination with other antimicrobials, Ch-arg packaging may offers some potential for limiting the growth of pathogenic bacteria in foodstuffs; however, further research is needed to enhance their anti-microbial performance.
Keywords
Cross-Contamination, foodborne Pathogen, Polymer Film, Shelf Life
1. Introduction
The conflict between consumers’ demand of minimally processed fresh food and an adequate shelf-life has slowly been challenged by the emergence of active packaging [1]. Active packaging incorporates antimicrobial substances and may play an important role in reducing contamination and delaying spoilage, thereby increasing the safety of meat products [2]. However, active substances in contact with food surfaces may be neutralized or diffuse quickly into the product, therefore, the direct surface application may not target food surfaces where most spoilage reactions occur [2]. It may be possible to increase the efficiency of antimicrobial packaging by developing films which contain antimicrobial agents. Previous research suggests that these films either allow the gradual release of active agents [3] or exert their effect when active sites contact bacteria directly. However, results of studies into the effectiveness of such films have so far been inconclusive [4, 5]. One inherently promising antimicrobial agent is chitosan, which is biodegradable, biocompatible and nontoxic [6]. Chitosan inhibits the growth of a wide spectrum of bacteria, pathogens and spoilage microorganisms, including E. coli O157 [7, 8], the pathogen of interest in this study. E. coli O157 is a frequent contaminant of meat, causing potentially fatal illnesses in humans such as haemorrhagic colitis and haemolytic uraemic syndrome [9, 10]. Packaged raw beef and chicken products represent a high-risk for contamination, since if not properly sealed, meat juices can escape and contaminate food preparation surfaces or other foodstuffs as well as providing a high-nutrient environment where pathogens such as E. coli O157 can multiply. This poses a major risk for hand-to-mouth pathogen transfer, cross-contamination during storage (e.g. in refrigerators) and on food preparation surfaces [11].
Although chitosan-based films have proven effective in food preservation [8], its application as a food preservative is limited by its insolubility at neutral pH. One functional derivative of chitosan, chitosan-arginine, has positively charged guanidinium side chains in neutral pH environments, leading to high solubility in water [12] and therefore is a good candidate for food preservation. The biocidal effect of this newly-developed chitosan derivative, however, is not well understood [12], and its application to meat environments under different storage temperatures remains unknown.
The blending of cellulose and chitosan may be useful for introducing antimicrobial activity into packaging film. Cellulose has a similar molecular structure to that of the chitosan backbone [13, 14] and has been used in edible films and coatings since the 1980s due to its suitable physical and chemical properties [15, 16].
This present study aimed to assay and characterise the antimicrobial effect of cellulose/chitosan-arginine (Ch-arg) film on E. coli O157, in both chicken and beef juice. This research sought to develop a new, more effective film combining cellulose with chitosan arginine.
2. Materials and Methods
2.1. Preparation of Chitosan Solutions
Chitosan-arginine (85% deacetylated with arginine constituting 25% of the total monomers on the polymer backbone; 41 kDa, purity > 99%) was synthesized by Synedgen Inc. (Claremont, CA, USA). A chitosan-arginine stock solution (1 g l-1) was made in distilled water and the solution sterilized by passage through a 0.2 μm syringe filter for storage and later use.
2.2. Cellulose/Chitosan-Arginine Film
A cellulose film-forming solution was prepared according to Gindl and Keckes (2007). Briefly, 3 g of microcrystalline cellulose (MCC) was dehydrated in ethanol and acetone, while 8 g of LiCl was dissolved in 100 ml N-dimethylacetamide (DMAc) [13]. After decanting the ethanol and acetone from the dehydrated cellulose, the LiCl/DMAc solution was slowly added to the cellulose over 5 min, before Ch-arg was drip-fed to the cellulose. Two contrasting Ch-arg doses were chosen based on preliminary experiments investigating optimal conditions for film production and antimicrobial activity [7]. The cellulose/Ch-arg mixture was then poured into 250 mm diameter Petri dishes and left under a fume hood for 24 h to form a thin film. The final concentration of Ch-arg in the cellulose matrix was either 0.5% (low concentration) or 1.5% (high concentration). Films containing no Ch-arg were used as a control.
2.3. Preparation of E. coli O157 Inoculum
A strain of E. coli O157 (strain 3704 Tn5 lux CDABE; [17]) was prepared from a fresh overnight Loria Bertani (LB) broth (Difco Ltd, Teddington, Surrey, UK; 37°C, 18 h, 150 rev min-1; [18]). The strain has proven to be non-toxigenic, due to the absence of toxin activity by Verocell assay, and toxin genes, but still accurately reflects the survival pattern of a toxigenic strain [17]. Cells were washed and concentrated by centrifugation as described in Avery et al. (2005) [19]. Enumeration of colonies (18 h, 37°C) was determined using the drop-plate method onto cefixime-tellurite-sorbitol-MacConkey agar plates (CT-SMAC; Oxoid, Basingstoke, UK) followed by incubation at 37°C for 24 h, and showed that the concentration of E. coli O157 in the inoculum was 8.93 log10 CFU ml-1.
2.4. Preparation and Characterization of Meat Juice
A total of six processed intact raw chickens were purchased from a commercial supermarket in Bangor, North Wales. Each chicken was placed in a sterile stomacher bag and thoroughly washed with sterile distilled water to obtain 600 ml of juice per chicken. The juice was then centrifuged for 10 min at 10,000 rev min-1 at 10°C, and sterilized by filtering through a 0.2 µm syringe filter.
Approximately 100 ml of beef juice was collected from three bovine meat joints (each ca. 5 kg), hung for 10-12 days in sterile plastic bags in a commercial butcher’s cold-room (4 ± 1°C; [18]). The juice was pooled together, and stored at 4°C for 24 h, before adding distilled water to make 1/10-strength beef juice. The juice was then centrifuged for 10 min at 10,000 rev min-1 at 10°C before being sterilized by passing through a 0.2 µm filter.
The chemistry of both meat juices was also characterized for pH (pH-209 meter; Hanna Instruments Inc., Woonsocket, RI), electrical conductivity (CDM210 meter; Jenway Ltd., Dunmow, UK) and dissolved organic phosphate (DOP). DOP was calculated by subtracting the amount of inorganic phosphate from total phosphate measured using a plasma-atomic emission spectrometer (ICP-AES; Varian Liberty Series, Franklin, MA, USA).
2.5. Antibacterial Testing in Chicken Juice
The films containing cellulose alone (control group) or cellulose with 0.5 or 1.5% chitosan-arginine were produced as previously described. The films were placed on the bottom of glass jars, to which 75 ml of meat juice and 4 ml of E. coli O157 inoculum were added, giving a final concentration of 8.93 log10 CFU ml-1. The jars were then covered with metal lids and stored at 20°C for 120 h (n = 3 for each juice-film combination). This temperature was chosen to reflect room temperatures when risks of microbial cross-contamination and growth are potentially highest. At 0, 2, 6, 12 and 24 h post-inoculation, and subsequently every 24 h until 120 h post-inoculation, 2 ml of juice was aseptically removed in triplicate from the bottom of the jars and analysed for E. coli O157 as follows: cell count via the drop plate method on CT-SMAC plates [7]; luminescence via a Tecan Infinite 200 PRO luminometer (Tecan Austria GmbH, Grödig, Austria) which displayed results in RLU (relative light units). The Live/Dead BacLightTM Bacterial Viability Kit (Molecular Probes Inc., Eugene, OR, USA) was used to ascertain the number of live and dead E. coli O157 cells according to Mauriello et al. (2005) [20]. Samples were viewed using a Zeiss Axioskop fluorescence microscope, with an average count taken over 15 microscope fields.
2.6. Antibacterial Testing in Beef Juice
Cell count and metabolic activity of E. coli O157 were determined as described for chicken juice above. In contrast to the chicken juice trial, a BacLightTM microplate assay was used to determine the percentage of live and dead bacteria using a Varian Cary Eclipse fluorescence microplate reader (Varian Inc., Palo Alto, USA). To calibrate the percentage of live E. coli O157 in the beef juice, the bacteria was grown until late log phase and then concentrated by centrifugation (10,000 g, 10 min). One ml of this suspension was added to a tube containing 20 ml ¼-strength Ringer’s solution (live bacteria) and the other 1 ml to a tube containing 20 ml 70% isopropanol alcohol (dead bacteria). Samples were pelleted by centrifugation (10,000 g, 10 min) in both tubes and subsequently re-suspended in 20 ml Ringer’s Solution. After repeating this process three times, the optical density was measured as 600 nm (approx. 0.6-0.8 OD600nm). Live: dead cells were then mixed in a variety of ratios, to be used for calibration of the BacLightTM method. E. coli O157 were stained following the manufacturer’s guidelines [21]. The data was analysed by calculating the percentage live E. coli O157, which was plotted as ratio of live in sample to live in pure E. coli O157 (calibration sample) in SigmaPlot (version 19) using the equation, f = a × exp(bx) (r2 = 0.998; a = 0.3566, b = 0.026).
2.7. Data Analysis
Data were analysed with SPSS v19.0 (IBM UK Ltd, Portsmouth, UK). A multivariate ANOVA was conducted to examine the effect of treatment (0, 0.5 and 1.5% Ch-arg) on cell count, RLU and percentages of culturable and live E. coli O157. Significant effects were identified using post-hoc Bonferroni-adjusted multiple comparisons test at p < 0.05, with simple planned contrasts used to examine the main effect of time. Significant interactions were followed up with repeated measures ANOVAs, with Tukey HSD post-hoc tests at p < 0.05. Twelve samples were analysed in total, comprising 4 conditions × 3 replications. Two-tailed independent samples t-tests were used to evaluate the differences in chemical characterization of beef and chicken juices.
3. Results and Discussion
3.1. Chemical Characterization of Meat Juice
The chemical characteristics of beef and chicken samples are shown in Table 1. In brief, a two-tailed independent samples t-test revealed pH was significantly lower in the beef juice, but that EC, inorganic and organic P of beef juice were significantly greater than that of chicken juice (all p < 0.003).
3.2. Effectiveness of C/Ch-arg Films on Reducing Cell Counts of E. coli O157
Cell counts of E. coli O157 in chicken juice incubated in the presence or absence of Ch-arg containing cellulose films are shown in Figure 1a. Until 6 h post-inoculation, E. coli O157 in the cellulose (control) treatment and the films treated with Ch-arg maintained similar growth patterns, increasing slightly from 7.7 log10 CFU ml-1 to 8.3 log10 CFU ml-1. After this, the population of E. coli O157 incubated in the presence of high concentrations of Ch-arg film significantly decreased. For the control treatment (no Ch-arg present) and the low Ch-arg film treatment, the population of E. coli O157 generally continued to grow after 12 h incubation, reaching 8.2 log10 CFU ml-1 by the end of the trial. Tukey HSD post-hoc tests showed no significant difference between the control group and the group treated with low Ch-arg concentration films (p = 0.76), but a significant difference between the control group and the high Ch-arg treatment (p = 0.02). Similarly, there was a marginally significant difference between the low and high Ch-arg treatments (p = 0.05).
The influence of Ch-arg films on the persistence of E. coli O157 in beef juice is shown in Figure 1b. In the absence of Ch-arg, the population of E. coli O157 remained relatively stable over the 120 h incubation period. Similarly, cell counts in the two Ch-arg treatments also remained relatively stable decreasing marginally from 8.4 log10 CFU ml-1 at time 0 to 8.3 by 120 h although this did not prove statistically significant (p = 0.31). Tukey HSD post-hoc tests revealed that there was a significant difference in E. coli O157 numbers between the low and high Ch-arg films at 120 h (p = 0.038). All other comparisons proved non-significant.
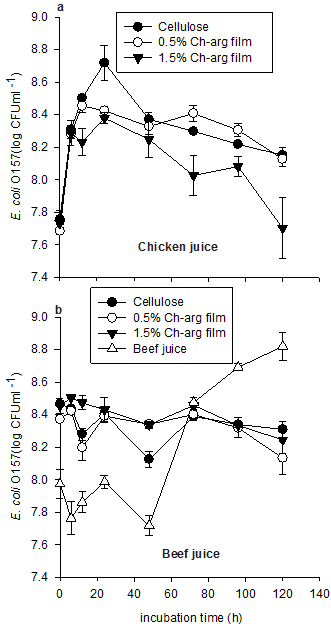
Figure 1. Cell counts (CFUml-1), of E. coli O157 in chicken juice (a) and beef juice (b) samples stored at 20°C. Values represent mean ± standard error (n = 3).
3.3. Effectiveness of Cellulose/Chitosan-Arginine Films on Cell Activity of E. coli O157
The effects of Ch-arg on the metabolic activity (as measured using bioluminescence) of E. coli O157 in chicken and beef juice are presented in Figure 2. In general, cell activity of E. coli O157 in all treatments groups declined during the 120 h incubation although this was much greater in the chicken juice than in the beef juice (p < 0.001). During this time period, there was no significant main effect of Ch-arg film concentration (p = 0.09 and p = 0.11 for chicken and beef juice, respectively).
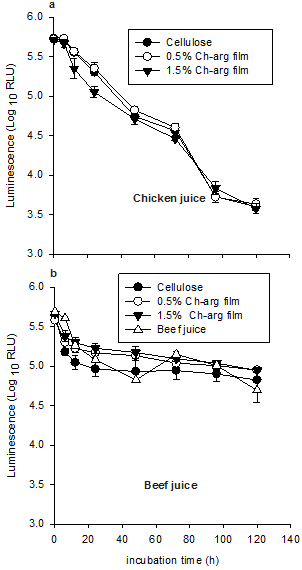
Figure 2. Metabolic activity as measured by luminescence (RLU), of E. coli O157 in chicken juice (a) and beef juice (b) samples stored at 20°C. Values represent mean ± standard error (n = 3).
3.4. Effect of Ch-arg Films on the Culturability and Proportion of Live E. coli O157 Cells
The percentage of live E. coli O157 in chicken juice was calculated to ascertain the effect of Ch-arg film on the culturability of E. coli O157 cells (Fig. 3). A multivariate ANOVA revealed no significant difference in the percentage of culturable E. coli O157 in samples treated with Ch-arg films and cellulose-only film (p = 0.65). Simple contrasts following a repeated measures ANOVA found the culturability of the cells to significantly increase in all treatment groups within 24 h of incubation (p = 0.004; from 1.1% to an average of 5.7%), before decreasing to a level of less than 2%.
In beef juice, the percentage of live cells generally decreased over time (p < 0.001, Fig. 3). In the cellulose-only and Ch-arg treatments, the percentage of live E. coli O157 decreased from 0.40-0.38% with no significant differences apparent between treatments.
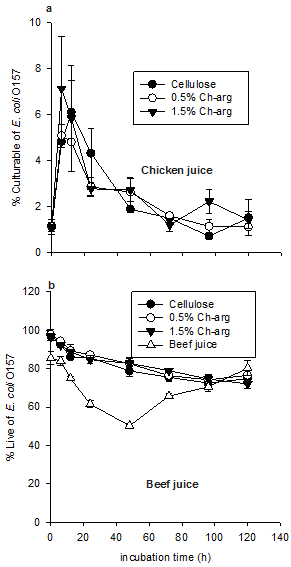
Figure 3. Percentage of culturable and live E. coli O157 in chicken (a) and beef juice (b) samples stored at 20°C. Values represent mean ± standard error (n = 3).
4. Conclusions
In the current study, a higher-concentration Ch-arg film was seen to exert a slight biocidal effect on E. coli O157 although the response was very weak in comparison to experiments where Ch-arg at similar concentrations was added directly to solution [7]. The response was only apparent, however, in chicken juice with no apparent effect observed in the beef juice medium. Results from the chicken juice experiment are consistent with previous research [22, 23] suggesting a concentration-dependence of the antimicrobial properties of chitosan and its derivatives in solution form, with an increased disruption of microbial cells observed at higher dose rates. Results from both chicken and beef juice experiments are also consistent with a recent study by Foster and Butt (2011), who found no inhibitory effect of unmodified chitosan films on other bacterial species commonly present in meat during 24 h of incubation [4]. Foster and Butt argued that chitosan chains in the films were unable to interact with the microbial cell walls, impeding antimicrobial effects [4]. However, in the current study the incubation time was longer (120 h), which may explain the antimicrobial effect of high concentration Ch-arg found in chicken juice and the modified chitosan possessed a higher charge density.
Results from the current study suggest that Ch-arg chains interact with meat juice cells in addition to microbial cell walls, reducing the chance of antimicrobial action. DOP levels were twice as high in the beef juice as in the chicken juice, suggesting the presence of more blood cells. Therefore, negatively charged phospholipids in blood cells may interact with positively charged Ch-arg, blocking the active sites of Ch-arg, causing the failure of chitosan to exhibit bactericidal properties. In support of this inhibitory effect, Devlieghere et al. (2004) also found antimicrobial activity to be inhibited when lipid was added to a chitosan solution [24].
Since Ch-arg lacks the cohesion to make gel alone, it was blended with cellulose. However, this may have reduced the surface exposure of the biocidal groups or prevented them from reaching a critical mass of the cell membrane surface. The use of higher concentrations are possible in other formulations, however, these would make film production uneconomic for use in meat preservation.
Acknowledgements
We would like to thank the department of Food Science and Technology, University of Tripoli, for funding this research.
References
- Dutta, J., Tripathi, S., Dutta, P. K., 2012. Progress in antimicrobial activities of chitin, chitosan and its oligosaccharides: a systematic study needs for food applications. Food Science and Technology International 18, 3–34.
- Restuccia, D., Spizzirri, U. G., Parisi, O. I., Cirillo, G., Curcio, M., Iemma, F., Puoci, F., Vinci, G., Picci, N., 2010. New EU regulation aspects and global market of active and intelligent packaging for food industry applications. Food Control 21, 1425–1435.
- Bastarrachea, L., Dhawan, S., Sablani, S. S., 2011. Engineering properties of polymeric-based antimicrobial films for food packaging. Food Engineering Reviews 3, 79–93.
- Foster, L. J., Butt. J., 2011. Chitosan films are not antimicrobial. Biotechnology Letters 33, 417–421.
- Lee, C. H., An, D. S., Park, H. J., Lee, D. S., 2003. Wide-spectrum antimicrobial packaging materials incorporating nisin and chitosan in the coating. Packaging Technology and Science 16, 99-106.
- Alishahi, A., Aider, M., 2012. Applications of chitosan in the seafood industry and aquaculture: a Review. Food and Bioprocess Technology 3, 817–830.
- Lahmer, R. A., Williams, A. P., Townsend, S., Baker, S., Jones, D. L., 2012. Antibacterial action of chitosan-arginine against Escherichia coli O157 in chicken juice. Food Control 26, 206-211.
- Dutta, P. K., Tripathi, S., Mehrotra, G. K., Dutta, J., 2009. Perspectives for chitosan based antimicrobial films in food applications. Food Chemistry 114, 1173–1182.
- Soon, J. M., Chadd, S. A., Baines, R. N., 2011. Escherichia coli O157:H7 in beef cattle: on farm contamination and pre-slaughter control methods. Animal Health Research Reviews 12, 197–211.
- Pennington, H., 2010. Escherichia coli O157. The Lancet 376, 1428–1435.
- Mattick, K., Durham, K., Dommingue, G., Jørgensen, F., Sen, M., Schaffner, D, W., Humphrey, T., 2003. The survival of foodbone pathogens during domestic washing-up and subseguent transfer onto washing –up sponges, kitchen surfaces and food. International Journal of Food Microbiology 85, 213–226.
- Tang, H., Zhang, P., Kieft, T. L., Ryan, S. J., Baker, S. M., Wiesmann, W. P., Rogelj, S., 2010. Antibacterial action of a novel functionalized chitosan-arginine against Gram-negative bacteria. Acta Biomaterialia 6, 2562–2571.
- Gindl, W., Keckes, J., 2007. Drawing of self-reinforced cellulose films. Journal of Applied Polymer Science 103, 2703–2708.
- He, L. H, Xue R, Yang, D. B., Liu, Y., Song R., 2009. Effects of blending chitosan with peg on surface morphology, crystallization and thermal properties. Chinese Journal of Polymer Science, 27, 501–510.
- Li, Z., Zhuang, X. P., Liu, X. F., Guan, Y. L., De Yao, K., 2002. Study on antibacterial O-carboxymethylated chitosan/cellulose blend film from LiCl/N, N-dimethylacetamide solution. Polymer 43, 1541–1547.
- Sangsuwan, J., Rattanapanone, N., Rachtanapun, P., 2008. Effect of chitosan/methyl cellulose films on microbial and quality characteristics of fresh-cut cantaloupe and pineapple. Postharvest Biology and Technology 49, 403–410.
- Ritchie, J. M., Campbell, G. R., Shepherd, J., Beaton, Y., Jones, D., Killham, K., Artz, R. R. E., 2003. A stable bioluminescent construct of Escherichia coli O157:H7 for hazard assessments of long-term survival in the environment. Applied and Environmental Microbiology 69, 3359–3367.
- Williams, A. P., McGregor, K. A., Killham, K., Jones, D. L., 2008. Persistence and metabolic activity of Escherichia coli O157:H7 in farm animal faeces. FEMS Microbiology Letters 287, 168–173.
- Avery, L. M., Killham, K., Jones, D. L., 2005. Survival of E. coli O157:H7 in organic wastes destined for land application. Journal of Applied Microbiology 98, 814–822.
- Mauriello, G., DeLuca, E., La Storia, A., Villani, F, Ercolini, D., 2005. Antimicrobial activity of a nisin-activated p; ,^# astic film for food packaging. Letters Applied Microbiology 41, 464-469.
- Molecular Probes, 2004. Live/Dead® BacLightTM Bacterial Viability Kits http://www.probes.invitrogen.com/media/pis/mp07007.pdf (accessed 5/10/12)
- Xiao, B., Wan, Y., Zhao, M., Liu, Y., Zhang, S., 2011. Preparation and characterization of antimicrobial chitosan-N-arginine with different degrees of substitution. Carbohydrate Polymers 83, 144–150.
- Wu, Y., Yu, S., Mi, F., Wu, C., Shyu, S., Peng, C., Chao, A., 2004. Preparation and characterization on mechanical and antibacterial properties of chitsoan/cellulose blends. Carbohydrate Polymers 57, 435–440.
- Devlieghere, F., Vermeulen, A., Debevere, J., 2004. Chitosan: antimicrobial activity, interactions with food components and applicability as a coating on fruit and vegetable. Food Microbiology 21, 703–714.

 (R. A. Lahmer)
(R. A. Lahmer)