| 1. | ||
| 2. | ||
| 3. | ||
Oxide Ceramics for Solar Hydrogen Production and Water Purification
Shahab Khameneh Asl1, S. M. Nassab2
1Department of Ceramic Engineering, Faculty of Mechanical Engineering, University of Tabriz, Tabriz, Iran
2Faculty of Mechanical Engineering, Tabriz Azad University, Tabriz, Iran
Email address
sshahab_kh@yahoo.com (S. K. Asl)
Shahab Khameneh Asl, S. M. Nassab. Oxide Ceramics for Solar Hydrogen Production and Water Purification. American Journal of Environmental Engineering and Science. Vol. 3, No. 2, 2016, pp. 43-47.
Abstract
Photocatalytic reaction is a challenging topic because it is an important solution to energy and environmental issues. Recently, many oxide composited powders for water splitting have been developed. For example, Pt/ZnO, TiO2 photocatalystwith a 3.2 eV band gap showed high activity for water splitting into H2 and O2 withan apparent quantum yield of 67% at near UV ranges. In this composite, two oxide couples as an electron relay system employing to increase the efficiency of hydrogen production. Moreover, highly efficient for solar hydrogen production in the presence of electron donors were developed by adding platinum.
Keywords
Photocatalysts, Water Splitting, Hydrogen, Coupled Oxides
1. Introduction
More than ninety percent of the world’s energy consumption is provided by non-renewable energy systems based on the wood, coal, oil and uranium. Environmental pollution is a problem with these sources. Collecting and converting solar energy at 1% efficiency, the solar radiation reaching the surface of earth. The main process for future energy supplies could be "photo hydrogen" production from water splitting [2]. Photocatalytic processes are cheap, clean and employ a renewable source which is sun light. In these processes photo irradiated semiconductors produced electron/hole pairs, which are applied in redox reactions. In these reactions, water is reduced and oxidized to hydrogen and oxygen [3,4].
The application of these type of materials in Advanced Oxidation Processes (AOP) is broad as Fujishima summarized for one of the most important photo catalysts; TiO2 in Figure 1.
Water splitting is a catalyst based reaction where the efficiency and rate of the reaction is related to many factors [6, 7]. The system selected for reaction depends on parameters like: type of reactor, temperature, flow, pressure, time, light source, catalyst content and type, additives and etc. These factors effect of kinetics of reaction. Scientists try to optimize each factor to increase the rate of reaction. The semiconductor that used as a catalyst is is dependent on its components and its properties. This includes the type of semiconductor, the component ceramics and microstructure. The microstructural parameters including the size, specific surface area, pore volume, pore structure, crystalline phase, and the exposed surface facets can exert significant influence on photocatalytic performance [6,8].
The modified semiconductor was prepared by doping with Fe, Zn, Cu, Ni, V, Mg, Be and Ni and or by impregnating with noble metals as Pt, Pd, Ir, Rh, Ru as co-catalysts or
preparing mixed oxides like TiO2, CuO, ZnO, NiO and CeO [9]. These catalysts mainly show higher photocatalytical activities [10] as well as potential applications in the water splitting. The main propose of the obtained metallic oxide has been related to the creation of oxygen vacancies in the TiO2 crystalline structure and mixed oxide improvement related to their ability to reduce the recombination rate of the photo produced e/h pairs [5].
Thus, the development of performance improvements by adjusting these factors remains the focus of photocatalysis research. In the present work, a nobel metal doped mixed oxide catalyst was prepared by wet chemical method and its photocatalytical activity in water splitting process calculated and evaluated and compared with its basic types.
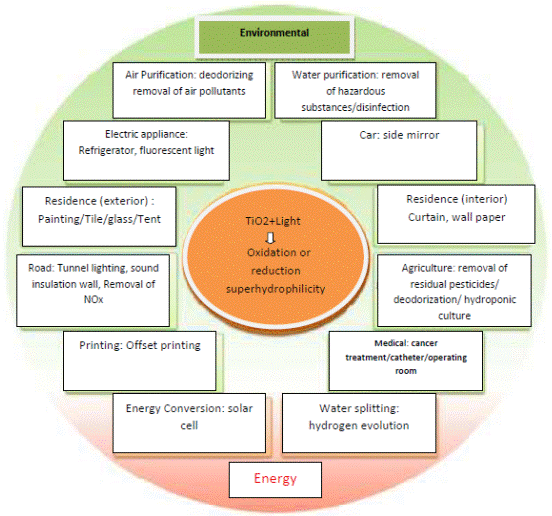
Figure 1. Application of TiO2 as a photo catalyst in different fields [5].
2. Experimental
Catalyst preparation
The nanostructured TiO2–ZnO samples were prepared by the sol–gel method using titanium isopropoxide (Aldrich 97%) and zinc acetate trihydrate (Merck 99%) as precursors: 10 mL of propanol (Kemetsan 99.5%) and 50 mL of distilled water containing the appropriated amount of zinc acetate to obtain solids with 1.0, 5.0 and10.0 wt. % Zn, were mixed and a few drops of HCl were added in order to obtain a pH = 2 in the solution. After, the solution washeated under reflux at 70°C; 50 mL of titanium isopropoxide was added drop wise and maintaining during 3 h under magnetic stirring until the gel was formed. Afterwards, the gel was dried at 70°C during 20 h. The solid was then ground to a fine powder in an agate mortar. The obtained powder was then heated at 550°C during 6 h in an air atmosphere using a heating rate of 5°C/min. The reheated product powder was ground again. As reference pure TiO2 or ZnO samples were prepared in the same way described above but without Zn or Ti precursor [11]. About 5 wt% of Pt added to catalyst by wet impregnation method by using chloroplalinic acid hexahydrate (H2PtCl6.6H2O, Aldrich) salt.
Catalyst characterization
Hydrogen adsorption (desorption) isotherms were obtained with a handmade manifold system containing high vacuum pump, pressure readout and hydrogen source [12]. Prior to the hydrogen adsorption, all the samples were outgassed overnight at 300°C. The Pt coverage was calculated from hydrogen adsorption isotherms. The specific surface area of the samples were derived from the BET Brunauer–Emmett–Teller) results, and the mean pore size diameter from the desorption isotherms using the BJH (Barrett-Joyner-Halenda) method. The crystalline size and phases of the obtained TiO2, ZnO and TiO2–ZnO powders were analyzed by X-ray diffraction (XRD) using internal calibration and Scherrer formula. EDX (Energy-dispersive X-ray spectroscopy) spectra were recorded with a analytical system coupled to a SEM microscope from Philips XL30. This microscope was also used to obtain the SEM images. The UV/Vis absorption spectra were obtained with a PG Instruments T80+ spectrophotometer.
Photocatalytic H2 production
The photoactivity for the hydrogen generation was evaluated using a homemade PyrexTM reactor of 200 mL containing an aqueous solution water–methanol (1:100 M ratio) and 0.2 g of catalyst. Irradiation was provided by a high pressure Hg pen-lamp (with aradiation of 254 nm and intensity of 100 watt). The amount of hydrogen produced was followed by gas chromatography using a gas chromatograph HP 4890. Before the carried out the H2 production using the batch reactor, a blank test was made without methanol and H2 was not produced. Our reactor is PyrexTM glass which blocks a portion of the UV region of light so, Pt used to increase the efficiency in near UV irradiation.
Results and discussion
In Figure 2 the XRD patterns of TiO2, ZnO and TiO2–ZnO mixed oxide are illustrated. The powder XRD patterns show anatase, rutle phases of titania and wurtzite of ZnO formed. It is identified by the maximum peaks at (101) at 2θ = 25.4° (anatase), 27.38° (rutile) and 36.19 (wurtzite) respectively. The results of Scherrer calculation summarized in Table 1, as seen, all powders in nano size, in mixed samples overlapping of peaks decrease the accuracy of calculation but overall crystalline size increased because of seeding effect [13].
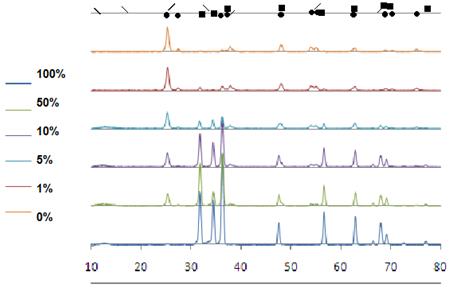
Figure 2. XRD graphs of samples from 2θ between 10-80 degree with different wt% of ZnO (•TiO2, ■ZnO, / zinc titanates, \precursors).
The specific surface area of the samples annealed at 550°C are summarized in Table 1, too. The results show that the BET specific surface areas of the TiO2–ZnO semiconductors are higher than that of the pure TiO2. As the amount of Zn increases a diminution in the specific surface area was observed from 50 to 80 m2/g then decreased to 30m2/gr for pure ZnO. The coverage of Pt on the surface of catalyst is one of the main factors in hydrogen production rates. Platinum as a noble metal not only increase the rate of reaction for electron transfer between bounds of semiconductor/metal, but also, introduce suitable sites on the surface to produce hydrogen from free radicals [12, 14]. As seen in the Table 1, the coverage of mixed powders is higher than pure ones, this can be as a result of being edge of two or three phases of powders.
Table 1. Powders specification.
| Name | Anatase (wt%) | Rutile (wt%) | ZnO (wt%) | Specific surface area m2/gr | Coverage % | EDX Ti/Zn ratio |
| TiO2 | 70 | 30 | - | 50 | 16 | 100/0 |
| TiO2-10ZnO | 65 | 25 | 10 | 65 | 23 | 10/1 |
| TiO2-5ZnO | 67 | 27 | 6 | 80 | 25 | 20/1 |
| ZnO | - | - | 100 | 30 | 7 | 0/100 |
The microstructure and EDX results indicate that the value of the content of the elements determined from the EDX spectra are similar to initial concentration the distribution of Zn in TiO2 matrix is randomly and fully dispersed. In Figure 3 micrograph pictures of the morphology of the samples are shown. It can be appreciated that the powder has high uniformity. Also it is shown in the micrographs cubic structure related to ZnO crystals.
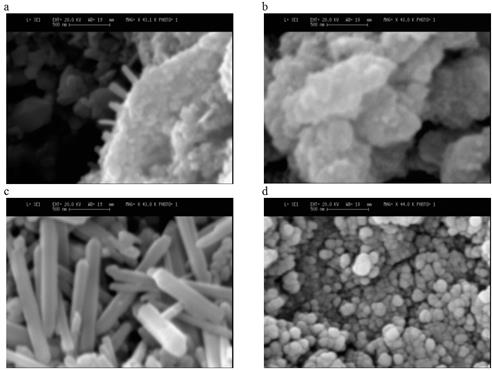
Figure 3. SEM images of a: TiO2-5ZnO, b: TiO2-10ZnO, c: ZnO, d: TiO2.
UV/Vis spectra were recorded to analyze the effect of secondary phase on adsorption edge of semiconductor and bang gap of photocatalyst. Figure 4 shows the UV/Vis spectra of the materials in solution. All samples exhibit an optical absorption below 450 nm, which can be attributed toelectron Ti–O transition of nanocrystalline TiO2. The results showa broad shift to the red region (3.12–3.03 eV) for the TiO2–ZnOsamples in comparison to TiO2 reference semiconductor (3.2/3.0 eV). Thus, the incorporation of ZnO to TiO2 only exerts small variations in the Eg energy band gap. This behavior could be as a result of electron/hole jump between near conduction and valance bounds of anatase, rutile and wurtzite or quantum size effect of small particles of ZnO on the surface of TiO2 (blue shift) [15].
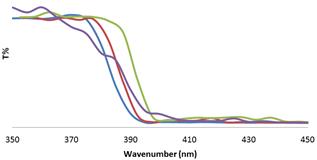
Figure 4. UV/Vis spectra of samples (green: TiO2, red: ZnO, blue: TiO2-5ZnO, violet: TiO2-10ZnO.
Fig. 5 shows the hydrogen production as a function of the irradiationtime for pure TiO2 and TiO2–ZnO photocatalysts. It can be seen that the hydrogen formation increases with the Zn content. The hydrogen production for TiO2 was 200 μmol/h.grcat, while for the TiO2–ZnO oxides it was 900 and 1200 μmol/hgr cat for TiO2–ZnO respectively. The mixed oxide more active results six times better than bare TiO2. ZnO shows no photo water splitting because of photocorrosion limitation. The increase in efficiency is a result of higher surface area, higher metal coverage and lower electron hole recombination by charge traps [16, 17].
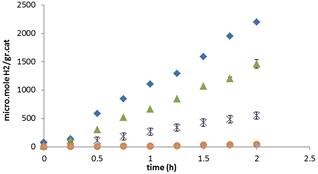
Figure 5. Graphs of H2 production, TiO2–ZnO samples, a: TiO2 (x), b: TiO2–5 ZnO (∆), c: TiO2–10ZnO (♦), d: ZnO (●).
3. Conclusions
The mixed oxide photocatalysts were prepared by wet chemical method and tested in the water splitting for the hydrogen production using a Pt, methanol and UV lamp in batch reactor. The effect of ZnO content in increasing the specific surface area and coverage of the powders was seen. It is showed that the preparation method allows the formation of the titania (anatase/rutile) and ZnO phases in the nano crystalline size. With the mixed oxides an activitysix times higher than that obtained with the pure TiO2 semiconductor was shown.
Acknowledgments
The authors thanks to Tabriz University for scholarships and Cactus Research group for some experimental works.
References