Heavy Metals and Polycylic Aromatic Hydrocarbon Concentrations in Fish Species from River Ororefe, in Ajanesan Oghara Town, Delta State, Nigeria
Peters D. E.*, Bala B. K., Abbey B. W.
Deparatment of Biochemistry, University of Port Harcourt Choba, Rivers State, Nigeria
Email address

(Peters D. E.)
*Corresponding author
Citation
Peters D. E., Bala B. K., Abbey B. W. Heavy Metals and Polycylic Aromatic Hydrocarbon Concentrations in Fish Species from River Ororefe, in Ajanesan Oghara Town, Delta State, Nigeria. Biochemistry and Molecular Biology. Vol. 1, No. 1, 2016, pp. 1-5.
Abstract
Study was carried out to determine the level of some heavy metals: copper (Cu), mercury (Hg), chromium (Cr), lead (Pb), zinc (Zn), cadmium (Cd) and nickel (Ni) and polycyclic aromatic hydrocarbon (PAHs) in Atlantic Tarpon, Megalops atlanticus and Lady fish, Elops saurus. Fish samples were collected from Ororefe river in Ajanesan Oghara town, Ethiope Local Government Area in Delta State. Atomic absorption spectrophotometer (AAS) was used to determine heavy metal concentration and agilant 6890N Gas chromatography (GC) to determine polycyclic aromatic hydrocarbon. Mean values of Pb, Hg, Zn and Cd in Megalops atlanticus (0.33mg/kg, 0.00mg/kg, 14.08mg/kg and 0.00mg/kg respectively and in Elops saurus (0.18mg/kg, 0.00mg.kg, 19.59mg/kg and 0.00mg/kg) were between the permissible limits of WHO and FEPA. Ni & Cr concentratons in Megalops atlanticus (3.69mg/kg, 2mg/kg), and (2.92mg/kg and 1.37mg/kg) in Elops saurus exceeded the permissible limit of WHO and FEPA. Cu concentration in Megalops atlanticus (2.79mg/kg) fell within the permissible limit but in Elops saurus (4.43mg/kg), it exceeded the permissible limit of WHO and FEPA. PAHs detected were benzo (g,h,i) perylene (0.22828 mg/kg), benzo (k) fluoranthene (0.0015mg/kg), fluorene (0.07845mg/kg) and naphthalene (0.13 to 1mg/kg), in Megalops atlanticus and benzo (k) fluoranthene (0.0745mg/kg), indeno (1,2,3–cd) pyrene (0.00214mg/kg), naphthalene (0.7458mg/kg), and pyrene (0.14216mg/kg) in Elops Saurus. Total PAHs concentration was higher in Megalops atlanticus (0.51281mg/kg) than in Elops Saurus (0.39103mg/kg) with higher value of high molecular weight (HMW) PAHs as compared with low molecular weight (LMW) PAHs which tend to be carcinogenic and mutagenic. The LMW – PAH/HMW ratio was less than one for both species, indicating anthropogenic origin of PAHs which tend to be carcinogenic and mutagenic on bioaccumulation. However, repeated and long-term consumption of fish species from river Ororefe could lead to toxicity in human due to bioaccumulation of these toxicants.
Keywords
Atomic Absorption Spectrophotometer (AAS), Gas Chromatography (GC), Atlantic Tarpon, Megalops atlanticus and Lady Fish, Elops saurus, Polycyclic Aromatic Hydrocarbon (PAHs)
1. Introduction
Pollution of water can come from both natural source and human activities. The sources of water pollution are classified into two types namely: point source pollution, discharged into the environemnt through pipes, sewers or ditches from specific places (mostly in urban areas), they are fairly easy to identify, monitor and regulate [1] and the non-point source pollution also called polluted run-off caused by land pollutants that enter bodies of water over large areas than at a single point. E.g. mining wastes, agriucltural run-off and construction sediments.Pollution reduces water quality which lead to harmful algal bloom and eutrophication, which can be harmful to both aquatic life and human health as they find their way into the food chain. Human health is largely determined by the diet.In our diet, sources of proteins, vitamins and unsaturated essential fatty acid can be gotten from fishes.Polycyclic aromatic hydrocarbons (PAHs) are class of persistent organic pollutants containing 2 or more fused benzene rings. They are known to be ubiqutous in both marine and terrestial environments [2]. Adverse effects of PAHs have also been observed in marine organisms and they include reduction, endocrine alteration malformation of embryo and larvae and DNA damage. Ingestion of contaminated food [3] and diffusion from water across their gills and skin [4] are the major routes of PAHs exposure to fish and other sea foods. Various anthropogenic activities in coastal areas have contributed to PAH contamination of coastal environments.Several carcinogenic PAHs including benzo (a) anthracene, benzo (a) pyrene, ideno-(1,2,3-c,d) pyrene have been reported in water and food samples. As a result, the accumulation in the environment has become an issue of global health concern, the principal route exposure to man, being the consumption of contaminated water, foods and marine products expecially fish [5-7].
Heavy metals refer to any metallic chemical element that has a relatively high density and it is toxic or poisonous at low concentration. They are natural components of the earth’s crust and cannot be degraded or destroyed. As trace elements, some heavy metals metals (e.g. copper, selenium, zinc) are essential to maintain the metabolism of the human body.However, when in relatively high conentration, they lead to poisoning, heavy metal poisoning could result for instance from drinking-water contamination (e.g. lead pipes), high ambient air concentrations over emission sources or intake via tie food chain [8]. Heavy metals tend to bio-accumulate. Bio-accumulation means an increase in the concentration of a chemical in a biologial organism over time compared to the chemical’s concentration in the environment. Nigeria crude oil has the following heavy metals contents: vanadium (1.0ppm), nickel (4.oppm), cadmium (0.10ppm) while copper, lead etc. are less than 0.10ppm.
2. Materials and Methods
2.1. Materials/Equipments
Atomic absorption spectrophotometer (AAS), agilant 6890N Gas chromatography (GC)
2.2. Sample Collection Identification
Samples were collected from Ororefe in Ajenesan of Ogharaefe village in Oghara town in Ethiope West Local Government Area of Delta State. Latitude 5.53° and 6.05° North and longitude 5.30° and 6.05° East. Samples were collected using a fishing net after which samples were washed and identified as Atlantic tarpon, Megalopsatlanticus and Lady fish, Elopssaurus.
2.3. Digestion
Samples were washed in clean water and dried using an oven and ground using a blender. 40ml of HNO3, 40ml of H2SO4 and 20ml of HCLO were measured out using a measuring cyclinder to prepare a stock solution of 100ml in a volumetric flask. 1g of the sample was weighed and poured into a conical flask. 2ml of the stock solution was poured into the conical flask containing the sample and heated with a hot plate in a fume cupboard until dense fumes appeared. It was cooled and filtered with distilled water into a 100ml volumetric flask and made up to mark with the water.
2.4. Polycyclic Aromatic Hydrocarbon Determination
Flame Ionization Detector (F.I.D.) gas chromatography was used (Agilant 6890N gas chromatography). Sample was poured into a 1litre separating funnel. 50ml of methylere chloride was poured to a sample bottle and was shook for 30 seconds to rinse the inner surface. The solvent was transferred to the separating funnel containing the sample and the sample was extracted by shaking the funnel for 2minutes with periodic venting to release excess pressure. The organic layer was allowed to separate for a miniumum of 10 minutes, the methylene extract was collected in a 250ml flask. The sample extracted was re-extracted using another 50ml of methylene chloride and a third extraction was performed in this manner. The combined extract was poured through a drying column containing packed cotton wool, anhydrous sodium sulfate and silica, the extract was collected into the vial and concentrated by blowing with nitrogen gas to 1.0ml. The extract was mixed with 1.0ml of the solvent and 1.0micro litre was injected into the flame ionization detector gas chromatography for analysis [9].
2.5. Heavy Metal Determination
Atomic absorption spectrophotmeter was used. Aspiration method was used. A hollow cathode of the desired metal was installed in the instrument lamp compartment. The wave length dial was set according to the metal of interest. The instrument was turned on, and the current suggested by the manufacturer was applied to the hollow cathode lamp. The instrument was allowed to warm up with the energy so as to stablize for 10-20minutes. Air was turned on and its flow rate adjusted to the specific demand for metals to give maxiumum sensitivity.Acetylene was turned on and its flow rate adjusted to maxiumum sensitivity for the metal being analyzed. the flame was ignited and allowed to stabilize for few minutes. The blank consisting of deionized water was aspirated and the instrument was zeroed and the standard solution was aspirated and the aspiration rate of the nebulizer was adjusted to obtain maxiumum sensitivity. The sample was analyzed and the concentration was read directly from the instrument display [10].
The flame was extingnuished by turning off the acetylene first, then the air. The final concentration was calculated using the formular:
Where D = dilution factor, R = AAS reading, V=final Wt – wegith/quantity of sample used.
3. Result and Discussion
Fish are often at the top of the aquatic food chain and many concentrate large amounts of heavy metals from the polluted water that build up by ingestion, ion-exchange of dissolved metals across lipophilic membranes and absorption on tissue and membrane surface [11].
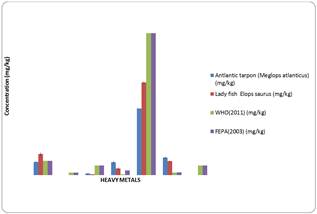
Figure 1. Concentrations of some heavy metals in some fish species from River Ororefe in Ajanesan Oghara Town, Delta State.
From figure 1, it is shown that the concentration of copper in Atlantic tarpon, Megalopsatlanticus (2.79.±0.10) falls between the permissible limit of WHO and FEPA while the copper concentration in Lady fish, Elopssaurus (4.43±0.34) is above the permissible limit of WHO and FEPA. High concentration of Cu in human leads to hormone imbalance, fatgiue, anxiety, joint pains, poor immunity, poor sleep, hypoglycemia, cancer and low histamine.
Mercury cocnentration in Atlantic tarpon, Megalops atlanticus (0.00±0.00) and in Lady fish, Elopssaurus (0.00±0.00) didn’t exceed the WHO and FEPA limit as well as Lead concentration (0.33±0.10) in Atlantic tarpon and (0.18 ± 0.03) in Lady fish.
Chromium cocnentration in Atlantic tarpon (2.74±0.26) and also in Lady fish (1.37±0.19) exceeded the permissible limit of WHO and FEPA and consumption of chromium in concentration above permissible limit will result in airway obstruction, lung, nasal or sinus cancer.
Cadmium concentration in Atlantic tarpon (0.00 ± 0.00) and in Lady fish (0.00±0.00) as well as zinc concentration in Atlantic tarpon (14.08 ± 0.10) and in Lady fish (19.59 ± 0.16) fell within the permissible limit of WHO and FEPA.
Nickel concentration in Atlantic tarpon (3.69 ± 0.21) and in lady fish (2.92 ± 0.09) fell above the permissible limit of WHO and FEPA and intake of such fish is associated with increased risk of lung cancer, ardiovascular disease, neurological deficits and high blood pressure.
Table 1. Individual quantities of some named PAH in Atlantic Tarpon.
| Name | Amount (in mg/kg) |
| Acenaphthene | ND |
| Acenaphthylene | ND |
| Anthracene | ND |
| 1,2-benzanthracene | ND |
| Benzo (a) pyrene | ND |
| Benzo (b) fluoranthene | ND |
| Benzo (g,h,i) perylene | 0.22828 |
| Benzo (k) fluoranthene | 0.07458 |
| Chrysene | ND |
| Dibenz (a,h) anthrancene | ND |
| Fluorenthene | 0.0015 |
| Fluorene | 0.07845 |
| Ideno (1,2,3-cd) pyrene | ND |
| Naphthalene | 0.13 to 1 |
| Phenanthrene | ND |
| Pyrene | ND |
| Totals | 0.51281 |
Table 2. Individual quantities of some named PAH in Lady fish.
| Name | Amount (in ppm) |
| Acenaphthene | ND |
| Acenaphthylene | ND |
| Anthracene | ND |
| 1,2-benzanthracene | ND |
| Benzo (a) pyrene | ND |
| Benzo (b) fluoranthene | ND |
| Benzo (g,h,i) perylene | ND |
| Benzo (k) fluoranthene | 0.07458 |
| Chrysene | ND |
| Dibenz (a,h) anthrancene | ND |
| Fluoranthene | ND |
| Fluorene | ND |
| Indeno (1,2,3-cd) pyrene | 0.00213 |
| Naphthalene | 0.17216 |
| Phenanthrene | ND |
| Pyrene | 0.14216 |
| Totals | 0.39103 |
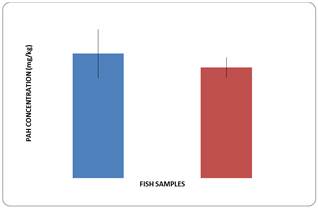
Figure 2. Total PAHs concentration in each fish sample was multiplied by 10.
Exposure pathways to PAHs in fish include bioconcentration from water across their gills and skins and ingestion of PAHs contaminated particulate matter along with food [3]. PAHs are lipophilic and so accumulated in the fatty tissues of fish following their uptake [2].
In Tables 1 and 2, allmost all the PAHs were not detected except Benzo (g,h,i) perylene (0.22828mg/kg), Benzo(k) fluoranthene (0.07450mg/kg), fluoranthene (0.0015mg/kg) and fluorene (0.07845mg/kg) detected in Atlantic tapon and Benzo (k) fluoranthene (0.07458mg/kg), ideno (1,2,3 –cd) pyrene (0.00213mg/kg), naphthalene (0.17216mg/kg) and pyrene (0.14216mg/kg) detected in Lady fish. Environmental Protection Ageny EPA has determined that benzo(b) fluoranthene, benzo (a) pyrene, benzo (k) fluoranthene, chrysene, dibenz (a,h) anthrancene, ideno (1,2,3 – cd) pyrene are probable carcinogenic to humans and anthracene, benzo (g,h,i) perylene, fluoranthene, fluorene, phenanthrene, pyrene and acenaphthylene are not classifiable as to humans carcinogenicity [12]. However both International Agency for Research on Cancer (IARC) and the Department of Health and Human Services (DHHS) classified ideno (1, 2, 3 –cd) pyrene as possible carcinogen [13]. PAHs determination in Atlantic tarpon and Lady fish, Benzo(a) pyrene which has been evaluated for carcinogenity by International Agency of Research on Cancer was not found in both fish samples [13].
Once PAHs enter the body they are metabolized in a number of organs (including liver, kidney, lungs), excreted in bile, urine or breast milk and stored to a limited degree in adipose tissue. PAHs induce expression of phase I and II metabolizing enzymes [14] and other enzymes catalyzing conjugation reactions [15]. PAHs undergo metabolic activation to diol-epoxides as we discussed before, which bind covalently to DNA. Afterwards, they form adducts or induce oxidative stress that provokes mutations. If DNA repair mechanisms are afflicted by the adduct formation rate the result is an accumulation of mutations in DNA that may induce carcinogenesis [16, 17].
From Figure 2 Atlantic tarpon showed higher amount of total PAHs as compared to Lady fish. The LMW – PAH/HMW ratio was less than one for both species, indicating anthropogenic origin of PAHs which tend to be carcinogenic and mutagenic on bioaccumulation. High molecular weight (HMW) PAHs are generally not actively toxic to aquatic organism and mutagenic while low molecular weight (LMW) PAHs have higher acute toxicity and this is enhanced by their high water solubility. [18].
4. Conclusion
Human health is largely determined by the diet. In our diet, proteins, vitamins and unsaturated essential fatty acid can be gotten from fishes. Heavy metals and PAHs are lipophilic and so accumulated in the fatty tissues, liver and kidney of fish following their uptake. Hence, consumption of fish species from river Ororefe (though these fishes are major sources of protein for rural dwellers) could lead to toxicity in human when repeatedly consumed over time due to bioaccumulation of these toxicants.
References
- Miller, G.T.J., (1996). Living the environemnt principles, connections and solution. Wadsworth publishing company U.S.A 9th ed. 431-432, 479-480.
- Bouloubassi, I., Fillaux. J., and Saliot, A., (2001). Hydrocarbons in surface and sediments from changjion (Yongtze river) estuary, East China Sea. Marine pollution bullet (2), 1335-1346.
- Meador, J.J., Sommers, F.C., Ylitalo, G.M. and Sloon, C.A., (1995). Altered growth and related psychological responses in Juvenile chinook Salmon (oncorhynchis tshawftsta) from direct exposure to polycyclic aromatic hydrocarbons (PAHs). Fish aquatic sci. 63:2364-2376.
- Gobas, F.A.P.C., Wilcockson, J.B., Russel, R.H., and Hffrer, G.D. (1999). Mechanism of biomagnification in fish under laboratory and field conditions. Envir.sci.technol. 33:131-141.
- Anyakora, C., Coker, H., Ogbeche, K., Ukpo, G., and Ogah, C., (2006). Hydrocarbon accumulation in fish as an index for level of hydrocarbon pollution. Journal of pharmaceutical science and pharmacy practice 8(12), 40-44.
- Gomez-Guillen, M., Gomez-Esala, J., Gimenez, B., and Monteno, P. (2009). Alternative fish species for cold-smoking process. International journal of food science technique. 44:1525-1535.
- Ajiboye, O., Yakub, .A., and Adom, T., (2010). A review of polycylic aromatic hydrocarbons and heavy metal contamination of fish from fish farms. Journal of applied science and environemntal managment 15(1), 235-238.
- Lawrence, E., Ekom, R.A. and Paul, M., (1991). Tmporal trends in heavy metal concentrations in the clam Egeria radata. (Bivalvia: Tellinacea: Donacidae) from the cross rivers, Nigera. RevHydrobiol 4(4):327-333.
- Saarnio, K.; Sillanpää, M.; Hillamo, R.; Sandell, E.; Pennanen, AS. & Salonen, R.O. (2008). Polycyclic aromatic hydrocarbons in size-segregated particulate matter from six urban sites in Europe.Atmospheric Environment, 42., 9087-9097.
- Draghici, C., Coman, G., Jelescu, C., Dima, C. & Chirila, E. (2010). Heavy metals determination in environmental and biological samples, In: Environmental Heavy Metal Pollution and Effects on Child Mental Development- Risk Assessment and Prevention Strategies, NATO Advanced Research Workshop, Sofia, Bulgaria.
- Agbozu, I.E., Ekweozor Ike and Opuene, K., (2007). Survey of heavy metals in the catfish synodontis cloria. Int. I environ.sci.technol.4(1):93-97.
- EPA. (2009). List of Priority Chemicals, In: United States Environmental Protection Agency Web Site, 03.02.2011, Available from http://www.epa.gov/osw/hazard/wastemin/priority.htm
- IARC. (2010). IARC monographs on the evaluation of carcinogenic risks to humans., In: International Agency for Research in Cancer Web site, 22.02.2011, Available from http://monographs.iarc.fr/ENG/Classification/index.php
- Shimada, T. (2006). Xenobiotic-metabolizing enzymes involved in activation and detoxification of carcinogenic polycyclic aromatic hydrocarbons. Drug Metabolic Pharmacokinetics, Vol. 21, No.4, (Aug), pp. 257-276, 1347-4367.
- Williams, J. A.& Phillips, D. H. (2000). Mammary expression of xenobiotic metabolizingenzymes and their potential role in breast cancer. Cancer Research, Vol. 60, No.17, (Sep 1), pp. 4667-4677.
- Bolognesi, C., Parrini, M., Aiello, C.& Rossi, L. (1991). DNA damage induced by 7,12- dimethylbenz[a]anthracene in the liver and the mammary gland of rats exposed to polycyclic aromatic hydrocarbon enzyme inducers during perinatal life. Mutagenesis, Vol. 6, No.2, (Mar), pp. 113-116, 0267-8357 (Print) 0267-8357 (Linking).
- Castorena-Torres, F., Bermudez de Leon, M., Cisneros, B., Zapata-Perez, O., Salinas, J. E.& Albores, A. (2008). Changes in gene expression induced by polycyclic aromatic hydrocarbons in the human cell lines HepG2 and A549. Toxicology In Vitro, Vol. 22, No.2, (Mar), pp. 411-421, 0887-2333 (Print).
- Uthe, J.F., (1991). Polycyclic aromatic hydrocarbons in the environment. Chem news 43(7):25-27.

 (Peters D. E.)
(Peters D. E.) 



