Utilization of Citric Acid Treated Corncob for an Efficient Removal of Mn(II) from Aqueous Solutions
Aref A. M. Aly1, Abdelhey A. Farrag2, Dalia Moatism3,
Sedky H. A. Hassan4
1Chemistry Department, Faculty of Science, Assiut University, Assiut, Egypt
2Geology Department, Faculty of Science, Assiut University, Assiut, Egypt
3Assiut University Mycological Centre, Assiut University, Assiut, Egypt
4Botany Department, Faculty of Science, New Valley Branch, Assiut University, Al-Kharja, Egypt
Email address
(A. A. M. Aly)
Citation
Aref A. M. Aly, Abdelhey A. Farrag, Dalia Moatism, Sedky H. A. Hassan. Utilization of Citric Acid Treated Corncob for an Efficient Removal of Mn(II) from Aqueous Solutions. American Journal of Environmental Engineering and Science. Vol. 3, No. 2, 2016, pp. 62-67.
Abstract
Citric acid pretreated corncob and the untreated one were used in this investigation to remove Mn(II) from aqueous solutions. The effects of pH, Mn(II) concentration, agitation time, adsorbent dose and particle size were evaluated. Equilibrium isotherms were analyzed by the Langmuir and Freundlich models and it was found that both models fit the experimental data (R2 values > 0.900). The kinetics of Mn(II) on both sorbents are in accord with the pseudo-second-order kinetics with a good correlation (R2 > 0.99).
Keywords
Citric Acid Treated Corncob, Mn(II) Adsorption, Adsorption Isotherms
1. Introduction
Industrial activities such as mining or metal processing discharge a large quantity of wastewaters bearing heavy metals that can be absorbed by living organisms and consequently enter the food chain to accumulate finally in the human body [1,2]. This means that the introduction of heavy metals in water is a serious environmental and public health concern due to the toxicity of these metals and their non-biodegradable nature.
Manganese is one of the undesirable elements in water supply. It is frequently found in waters containing iron. The presence of Mn(II) in water leads to some changes in appearance, taste, odor or color of water. It was reported that manganese causes neurological disorders in human being when ingested or inhaled at high concentrations (> 10mg/day) [3]. There is a variety of conventional methods to eliminate toxic metal ions from aqueous solutions. These include reduction and chemical precipitation [4], membrane filtration, ion exchange, liquid extraction or electrodialysis [5,6]. However, these methods have high operating costs and low feasibility for small scale industries or ineffective, especially when the metal concentration is higher than 100 ppm [7]. Therefore, research for efficient, eco-friendly, and inexpensive Mn(II) adsorption has been initiated. Metal removal by biomass is a complex process that depends on the chemistry of the metal ions, composition of biomass, and physicochemical factors such as pH, temperature, contact time, ionic strength, and metal concentration.
In this paper we investigate the factors affecting the removal of Mn(II) from aqueous solutions using citric acid treated corncob as an adsorbent with a comparative study on the untreated corncob for Mn(II) absorption. Absorption of Mn(II) on untreated corncob was already reported in the literature [8a], however, some aspects for Mn(II) adsorption on this sorbent need a reinvestigation. Mn(II) adsorption succeeded also on some other agricultural wastes [8b, 8c].
2. Materials and Methods
2.1. Chemicals, Biosorbents and Equipment’s
All chemicals used in this investigation were of analytical grades. The corncobs used in this study were brought from a local farm in Assiut, Republic of Egypt. The corncobs were washed with distilled water, shredded and oven dried at 70°C. The dried corncobs were sieved and particles of size 1mm were used for all experiments. The treated corncob was treated with citric acid, then oven dried at 70°C. A spectrophotometer of the type Evolution 300-UV-Visible was used for manganese (II) determination using perpurine as a complexing agent in the presence of a surfactant.
2.2. Effect of Contact Time on Biosorption Experiments
The treated or untreated corncob biosorbent (1 g/L) was suspended in 50 ml of Mn(II) solutions (50 mg/L) in 100 mL flasks. The suspensions were shaken (150 rpm) at 30°C. The pH of the solution was adjusted to 6.0 and samples were taken from the solutions at desired time intervals from 0-60 minutes and were subsequently centrifuged at 10,000 rpm for 5 minutes. Then, the Mn(II) concentration in the resulting supernatant was determined.
2.3. Effect of pH on Biosorption
The impact of solution pH on Mn(II) biosorption was investigated using the two biosorbents and conducted at different pH values (ranging from 2.0–8.0) containing 50 ml of metal solution. The pH adjustment was done with the addition of either 0.1M NaOH or 0.1M HCl.
2.4. Adsorption Experiments
In this study, the biosorption isotherms of Mn (II) were obtained at a pH value of 7.0. The sorption experiments were carried out using 1g of each biosorbent in conjunction with different concentrations of Mn (II) in 50 mL of deionized water. The solutions were shaken at 200 rpm for 30 minutes until equilibrium was attained. Then, the samples were centrifuged at 10,000 rpm for 5 minutes and the Mn (II) concentration in the supernatants was measured spectrophotometrically. The experiments were conducted at room temperature (30 ± 2°C). All the adsorption experiments were performed in duplicate.
2.5. Data Evaluation
The specific metal biosorption was calculated using the following equation:
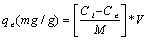 (1)
(1)
where qe is the specific metal biosorption (mg metal /g biomass), V is the volume of metal solution (L), Ci and Ce are the initial and equilibrium concentration of metal (mg metal /L), respectively, and M is the dry weight of the biomass (g).
3. Results and Discussion
The following operational parameters were studied in details to show their effects on the percentage removal or adsorption capacity of Mn(II) into untreated and citric acid treated corncob.
3.1. Effect of pH
The pH of solution can influence the adsorption efficiency of metal ions onto the adsorbent sites. So, the effect of solution pH on the adsorption percentage of Mn(II) on both untreated and treated corncob was investigated at various pH values. Close inspection of the two curves (Fig. 1) reveals that while the percentage removal of Mn(II) using the untreated corncob increases with increasing the pH value, on the contrary it decreases continuously in case of citric acid pretreated corncob. For the first behavior, the lower adsorption at acidic pH is attributed to the presence of excess H+ ions competing with the Mn(II) ions for adsorption sites. Increasing the solution pH values reduces the H+ ions on the surface and increasing the OH- ions, thus enabling an electrostatic attraction between the positive Mn(II) and the negative change sorbents sites [9]. For the citric acid treated corncob, however a decrease in percentage removal of Mn(II) was noticed. This may be rationalized by assuming that Mn(II) may be complexed to the citric acid leading to a progressive detachment of citric acid from corncob [10]. Investigation of the various parameters thus should be performed at lower pH's in case of citric acid pretreated corncob.
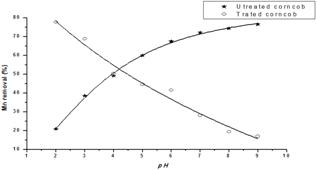
Fig. 1. Effect of pH on Mn(II) adsorption on treated and untreated corncob.
3.2. Effect of Mn(II) Concentration
The effect of Mn(II) concentration on the adsorption capacity (mg/g) of Mn(II) onto the untreated and citric acid treated corncob was studied where it is found that removal of the Mn(II) ions depends on the their concentration (Fig. 2). The initial metal ion concentration provides the necessary driving force for adsorption, thus facilitating the overcome of the resistance to the mass transfer of Mn(II) ions between aqueous and solid phases [11]. Adsorption of Mn(II) ions on untreated and citric acid treated corncob proceeds with almost the same trend.
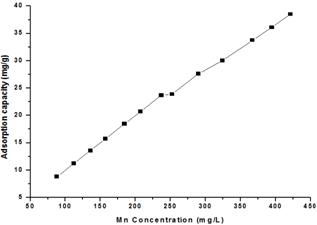
Fig. 2. Effect of Mn(II) concentration on the adsorption process onto citric acid treated corncob.
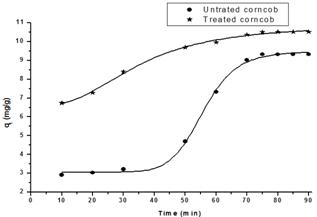
Fig. 3. Effect of agitation time on Mn(II) adsorption on untreated and citric acid treated corncob
3.3. Effect of Agitation Time
Contact time is one of the important parameters for a successful biosorption application. Fig. 3 illustrates clearly the effect of agitation time on the adsorption process. The adsorption capacity of untreated and citric acid treated corncob towards the Mn(II) ions show some differences of the two curves, although they exhibit the same trend. The adsorption capacity increases in the range 40-70 minutes for the untreated corncob (where from 10-30 minutes the adsorption capacity is almost constant). Afterwards the adsorption attains a constant value. For the acid treated corncob, there is a gradual increase in adsorption capacity from 20-60 minutes then the adsorption becomes almost constant. The uptake rate is supposed to be greatly affected by the rate at which Mn(II) ions diffuse from the surface to the interior sites of the adsorbent. The initial fast uptake is likely due to the high initial Mn (II) concentration and empty metal binding sites on corncobs (treated and untreated one). The slower subsequent phase is likely due to the saturation of metal binding sites. Therefore, one can conclude that the appropriate equilibrium time for measurements is reached at 60 minutes. The data obtained from this experiment was further used successfully to evaluate the kinetics of the adsorption process.
3.4. Effect of Sorbent Dose
The effect of the weight of untreated corncob and citric acid treated corncob on the percentage removal of Mn(II) ions is depicted in Fig. 4. Different weights of the sorbent were investigated ranging from 0.25 to 3.5 g. The maximum percentage adsorption of Mn(II) for untreated and acid treated corncob was specified when 1g of the sorbent was used. Above this quantity no appreciable change in percentage removal was noticed. This may be attributed to the saturation of sorption sites at higher sorbent dosages.
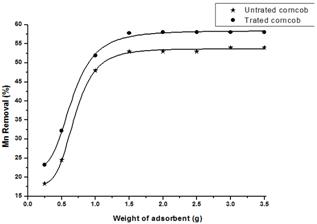
Fig. 4. Effect of sorbent dose on the Mn(II) adsorption on untreated and treated corncob.
3.5. Effect of Sorbent Particle Size
The effect of particle size of untreated and treated corncob on the percentage removal of Mn(II) is demonstrated in Fig. 5. The figure reveals a noticeable increase in the percentage removal of manganese with decreasing the particle size. Of course, the decrease in particle size would produce an increase in surface area of the corncob and consequently an increase in percentage removal. In addition to this surface adsorption of Mn(II), there is the intraparticle diffusion that occurs from the outer surface into the pores of corncob, and the larger the sorbent particle size, the greater is the diffusional resistance to mass transfer. Furthermore, there is a variety of factors that are also responsible for the lowering in adsorption capacity when the sorbent particle size increases. These may include the diffusional path length, mass, contact time and blockage sections of the particles [12].
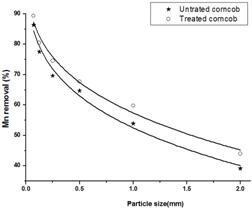
Fig. 5. Effect of sorbent particle size on Mn(II) adsorption on untreated and treated corncob.
3.6. Adsorption Isotherms
Adsorption isotherms are important to describe the interaction between the metal ions and the sorbent. The data were analyzed by the two most commonly used isotherm models, namely the Langmuir and Freundlich isotherms.
3.6.1. Langmuir Isotherm Model
This model suggests that sorption of metal ions proceeds via mono-layer adsorption on a homogenous sorbent surface, but without interaction between adsorbent and adsorbate ions.
The linear form of the Langmuir model is expressed as follows:
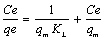 (2)
(2)
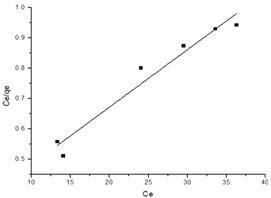
where Ce and qe are the concentration at equilibrium (mg/L) and the amount of Mn(II) ions adsorbed at equilibrium (mg/g), respectively, KL (L/g) is the Langmuir constant and is related to the adsorption energy and qm (mg/g), the maximum adsorption capacity. The maximum adsorption capacity qm, and KL can be determined from the linear form of Langmuir equation (Eq 2) .The plot of Ce/ qe vs. Ce is shown in Fig. 6.
A good fit by the Langmuir model indicates that the adsorption of Mn(II) could be characterized by a monolayer formation of the metal ion on the surface of the corncobs (treated or untreated) and belongs to a single type phenomenon with no interactions between sorbed metal ions. The maximum adsorption capacities calculated from Langmuir adsorption isotherm for untreated and treated corncobs were 43.8, and 52.6 mg/g, respectively.
3.6.2. Freundlich Isotherm Model
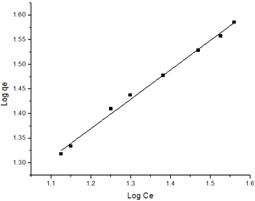
The Freundlich equation is based on a heterogeneous system and involves a multilayer adsorption. The linear equation is represented by equation 3.
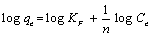 (3)
(3)
where 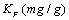 and n are the Freundlich constants corresponding to the adsorption capacity and adsorption intensity, respectively. Fig. 7 illustrates the Freundlich plot of log qe vs. ln Ce. Table 1 contains the results of Langmuir and Freundlich parameters. The data indicate that both models fit the experimental results, as they have R2 values > 0.90. However, the Freundlich model possesses a higher correlation coefficient than that of Langmuir. Because the
and n are the Freundlich constants corresponding to the adsorption capacity and adsorption intensity, respectively. Fig. 7 illustrates the Freundlich plot of log qe vs. ln Ce. Table 1 contains the results of Langmuir and Freundlich parameters. The data indicate that both models fit the experimental results, as they have R2 values > 0.90. However, the Freundlich model possesses a higher correlation coefficient than that of Langmuir. Because the 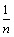 value is lower than one, so the Freundlich isotherm is favorable
value is lower than one, so the Freundlich isotherm is favorable 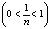 for adsorption of Mn(II). The magnitude of KF shows a higher uptake of Mn (II) using citric acid treated corncob (4.51 mg/g) compared to untreated corncob (1.98 mg/g).
for adsorption of Mn(II). The magnitude of KF shows a higher uptake of Mn (II) using citric acid treated corncob (4.51 mg/g) compared to untreated corncob (1.98 mg/g).
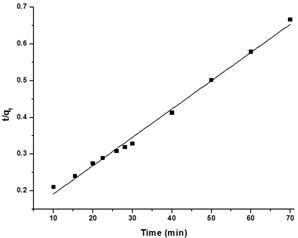
Fig. 8. Pseudo-second-order kinetics of citric acid treated corncob.
3.8. Intraparticle Diffusion Study
An empirical relationship was developed by Weber-Morris [13] that is common to most processes in which the uptake of sorbate is almost proportional to t1/2 according to equation 6.
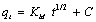 (6)
(6)
where Kid is the intraparticle diffusion rate constant (mg/g min1/2) and qt (mg/g) is the amount of Mn(II) adsorbed at time t (min). A plot of qt vs. t1/2 should be a straight line with Kid as the slope and C as the intercept. The intercept C is related to the thickness of the boundary layer, where larger intercept means a greater boundary layer effect [14]. Fig. 9 demonstrates the plot of Mn(II) adsorbed amount qt vs. t1/2 for both treated and untreated corncob. The two plots reveal that the process of Mn(II) adsorption occurs at two separate regions with two intersecting straight lines. The deviation of the straight lines from the origin points out to the difference in the rate of mass transfer in the initial and final stages of adsorption. The first straight portion is suggested to be associated with the macropores diffusion process, while the second linear portion can be ascribed to the micropore diffusion process. The intra particle diffusion parameters are cited in Table 3.
Table (3). Intraparticle diffusion model parameters for adsorption of Mn(II) on corncob.
| Untreated corncob | Treated corncob |
| | R2 | C | Kid | R2 | C | Kid |
| First Stage | 0.88 | 0.35 | 1.53 | 0.93 | 5.84 | 0.85 |
| Second Stage | 0.93 | -- | 0.31 | 0.93 | -- | 0.08 |
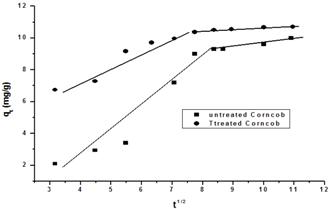
Fig. 9. Intraparticle diffusion of untreated and treated corncob.
Finally, comparing our results with those reported on adsorption of Mn(II) on untreated corncob, the following comments should be mentioned:
1. While our results reveal an increase in percentage removal of Mn(II) on untreated corncob by increasing pH of solution, the literature results [8] report, on the contrary, a decrease in percentage uptake.
2. The effect of Mn(II) concentration as well as agitation time and sorbent dose on its adsorption capacity are more or less as those reported in the literature.
3. Effect of particle size of the untreated corncob was not investigated in the previous studies and is included in this work.
4. Higher maximum adsorption capacity is obtained on untreated corncob in this work (43.8 mg/g) compared with that reported (6.54 mg/g) based on Langmuir model.
5. No data was found regarding intraparticle diffusion of Mn(II) on untreated corncob and is studied in this work.
6. While in this work the pseudo-second-order kinetic model is the best one that fits the experimental data (for untreated and treated corncob), the literature data [8] reported the pseudo-first order to be the proper model.
4. Conclusions
The study demonstrates that citric acid pretreated corncob is an effective sorbent for Mn(II) from aqueous solutions and it displays even higher removal efficiency than the untreated one. So, this investigation suggests the treated corncob as a low cost and ecofriendly candidate for removal of Mn(II) from aqueous solutions.
References
- E. Baldman, L. Erijman, F. L. P. Pessoa, S. G. F. Leite, Continuous biosorption of Cu and Zn by immobilized waste biomass Sargassum Sp. Process Biochem., 36 (2001) 869-873.
- K. S. Low, C. K. Lee, S. C. Liw, Sorption of cadmium and lead from aqueous solution by spent grain, Process Biochem, 36 (2000) 59-64.
- USEPA, "Integrated Risk Information System (IRIS) on Manganese" National Center for Environmental Assessment (1999), available athttp://www.Epa.gov/iris/subst/0373.htm.
- O. J. Esalah, M. E. Weber, J. H. Vera, Removal of lead, cadmium and zinc from aqueous solutions by precipitation with sodium di-(n-octayl) phosphinate, Can. J. Chem. Eng, 78 (2000) 948-954.
- L. Canet, M. Ilpide, P. Seta, Efficient facilitated transport of lead, cadmium, zinc and silver across a flat sheet-supported liquid membrane mediated by lasalacid A. Sep. Sci. Technol., 37 (2002) 1851-1860.
- A. K. Chakravarti, S. B. Chowdhury, S. Chakrabarty, T. Chakrabarty. D. C. Mukberjee, Liquid membrane multiple emulsion process of chromium (VI) separation from wastewaters. Colloids Surfaces A: Physicochemical Engineering Aspects, 103 (1995) 59-71.
- P. Miretzky, A. Saralegyi, A. F. Cirelli, Simultaneous heavy metal removal mechanism by dead macrophytes, Chemosphere., 62 (2006) 247-254.
- a)A. I. Adeoyun, A. E. Ofudje, M. Iowu, S. O. Kareen, Equilibrium, kinetic, and thermodynamic studies of the biosorption of Mn(II) ions from aqueous solution by raw and acid treated corncob biomass, BioResources 6 (2011) 4117-4134. b) S. A. Ahmed, A. M. El-Roudi, A. A. A. Salem, Removal of Mn(II) from ground water by solid wastes of sugar industry, J. Environ. Sci. Technol., 8(2015)338-351. c) N. Esfandiar, B. Nasernejad, T. Ebadi, Removal of Mn(II) from groundwater by sugarcane bagasse and activated carbon (a comparative study): Application of Response Surface Methodology (RSM), J. Ind. Eng. Chem., 20 (2014) 3726-3736.
- F. Mureed, R. Nadeem, A. Mehmod, Biosorption of zinc by chemically modified biomass of corncob (Zeamays L.), Middle-Eeast J. Scient. Res., 11 (2012) 1226-1231.
- F. A. Dawodu, K. G. Akpomie, Simultaneous adsorption of Ni(II) and Mn(II) ions from aqueous solution into a Nigerian kaolinite clay, J. Mater. Res. Technol., 3 (2014) 129-141.
- S. Gupta, B. V. Babu, Utilization of waste product (tamarind seeds) for the removal of Cr(VI) from aqueous solutions: Equilibrium, Kinetics and generation studies, J. Environ. Managem., 90 (2009) 3013-3022.
- A. Shukla, Y. H. Zhang, P. Babey, J. L. Margrave., S. S. Shukla, The role of sawdust in the removal of unwanted materials from water, J. Hazard. Mater., B95 (2002) 137-152.
- W. J. Weber Jr, J. C. Morris, Kinetics of adsorption on carbon from solution, Jessant Engg. Div ASCE, 89 (1963) 31-59.
- K. Kannan, M. M. Sundaram, Kinetics and mechanism various carbons: A comparable study, Dyes and Pigments, 51 (2001) 25-40.

 (A. A. M. Aly)
(A. A. M. Aly)