A Review on Nano CaCO3 and Flyash SBR Nano Composites
S. Dwarakesh*, G. Siva Kumar
Department of Mechanical Engineering, Panimalar Engineering College, Chennai, India
Email address

(S. Dwarakesh)

(G. S. Kumar)
*Corresponding author
Citation
S. Dwarakesh, G. Siva Kumar. A Review on Nano CaCO3 and Flyash SBR Nano Composites. AASCIT Journal of Nanoscience. Vol. 2, No. 2, 2016, pp. 13-17.
Abstract
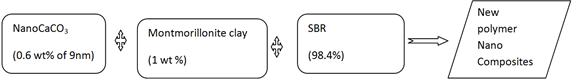
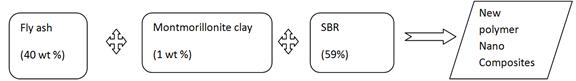
Nano CaCO3 of size (9 nm), Commercial CaCO3, were added from 0.4 wt% to 0.5 wt% in styrene butadiene Rubber (SBR) separately. These nanocomposites were compounded and moulded. Mechanical and thermal properties were studied. These result were compared to fly ash filled SBR. There was an improvement in properties of rubber due to uniform dispersion of nano CaCO3 particles in the matrix. Then three new scheme of combing nano CaCO3, montmorillonite clay, Flyash were added in different ratios to SBR. This new Scheme of mixing Nano CaCo3, montmorillonite clay will give a new nano composites with increased Mechanical and Thermal properties. This can improve the burning resistance and absorbs the heat of burning which improves the flammability of rubber nano composites.
Keywords
Mechanical and Thermal Properties, Nano CaCo3, Flyash, Montmorillonite
1. Introduction
Polymer/clay nanocomposites are a class of hybrid materials composed of organicpolymer matrix in which inorganic particles with nanoscale dimension are embodied. At this scale, the inorganic fillers improve dramatically the properties of polymer even though their amount is small. These nanocomposites exhibit improved modulus, lower thermal expansion coefficient and gas permeability, higher swelling resistance and enhanced ionic conductivity compared to the pristine polymers presumably due to the nanoscale structure of the hybrids and the synergism between the polymer and the silicate. [1], [2], [3] Preparing nanocomposites by intercalating layered silicates has proved to be a versatile approach to diminish the length scale of component phase.
Styrene-butadiene rubber (SBR) is widely employed in the automobile industry and for other rubber items. Great attention has been attached to modifying the combustion characteristics of SBR to reduce any potential hazard the material may present. Some approaches have been used to prepare rubber compositions with decreased tendency to burn, comprising:
1) Compounding with flame-retardant additives, such as metal hydroxide, phosphorus-containing additives, and chlorinated compounds;
2) post reaction of the elastomer with specific reagents;
3) preparing modifiedrubber by copolymerization with a small amount of special co monomers;
4) Modifying the cure system. In practice, it is very important that the flame retardant compounding formula doesn’t severely impair the mechanical properties of the rubber.
However, it is difficult to optimize among performance, burning behavior, and cost when the flame-retardant rubber is prepared using the above mentioned method. A technique that is low cost, halogen free, and has no negative effect on mechanical properties is required. Polymer layered silicate nanocomposites present unique properties compared with their pure polymersor conventional filled polymers 10–15 in aspects such as improved mechanical properties, decreased gas permeability, and increased solvent resistance. Moreover, this kind of nanocomposite usually exhibits increased thermal stability and improved flame retardancy, which are important characteristics for high-temperature applications.
1.1. Nano CaCO3
Calcium Carbonate is a naturally occurring inorganic biomaterial. It is a white, insoluble solid occurring naturally as chalk, limestone, marble, and calcite, and is the main component of shells of marine organisms, snails, pearls, and eggshells. It is a white powder or a stone. Calcium carbonate exists in three polymorphs: calcite, aragonite, and vaterite. Aragonite has got enormous research attention because of its biocompatible properties Among these three polymorphs, calcite is thermodynamically stable Even though calcite is the most stable polymorphism of calcium carbonate, aragonite has higher density and hardness which make it very suitable material in plastic, paper, glass, fiber and other industry. Among biological minerals, calcium carbonate has a special place since it is the main constituent of bones and shells. In both materials, the inorganic mineral is associated with biopolymers. Calcium Carbonate bio deposits exhibit variation in crystal size, shape, morphology, texture and aggregation. Calcium Carbonate nano particles (<100 nm) have shown many unique properties compared to regular particles.
The mechanical and thermal properties of polymers and composites structures can be improved through the use of various kinds of fillers. Calcium carbonate is one of the important inorganic fillers which is widely used in paints, plastic, and rubber industry. Micro sized calcium carbonate may have negative impact on the mechanical performance of polymers. It was reported that mechanical properties of polymer could be improved by adding nano-sized fillers. Of these fillers, nano-sized calcium carbonate (nano CaCO3) particles have attracted considerable attention and play effective role in many composites system. Nano CaCO3 is the cheap and commercially available, and has a low aspect ratio and large surface area [1]. Nano CaCO3 show superior physical and mechanical behavior over their conventional micro CaCO3 [1].
Wu et al [1] investigated the mechanical properties of nano composites of calcium carbonate, and found that elongation at break, young’s modulus, impact strength, mechanical strength increased with increasing the nano CaCO3 loading.
1.2. Fly Ash
Fly ash, an industrial waste, can be used as a potential filler material in polymer matrix composites because it is a mixture of oxide ceramics. It improves the physical and mechanical properties of the composites [4]. Reduction in filler size gives better enhancement in properties due to uniform distribution of particles in polymer matrix and increases degree of cross linking of matrix [4]. A mechanical property such as tensile strength, impact strength and hardness of PMC is enhanced with the addition of smaller size filler materials. The use of fly ash as a reinforcement in polymer matrices gets strong support from a discipline such as civil engineering. [4]
1.3. Styrene-Butadiene Rubber (SBR)
Styrene-butadiene or styrene-butadiene rubber (SBR) describes families of synthetic rubbers derived from styrene and butadiene. These materials have good abrasion resistance and good aging stability when protected by additives. In 2012, more than 5.4 million tones of SBR were processed worldwide. Styrene butadiene rubber is widely used in rubber industries, auto mobile industries. SBR plays an important role in automobile industries. About 50% of car tires are made from various types of SBR. The styrene/butadiene ratio influences the properties of the polymer: with high styrene content, the rubbers are harder and less rubbery.
1.4. Nano CaCO3+SBR
Nano CaCO3 is used as main filler in styrene butadiene rubber. When SBR is mixed with nano CaCO3 filler, glass transition temperature, tensile strength, elongation at break, modulus at 300% elongation, specific gravity and hardness, swelling index, flame retardency (FR) and abrasion resistance index (ARI) was increased.
1.5. Flyash+SBR
Fly ashfilled SBR shows a increase in Tg, tensile strength, elongation at break, modulus at 300% elongation, specific gravity and hardness, swelling index, flame retardency (FR) and abrasion resistance index but were not as much as nano CaCO3 filled SBR.
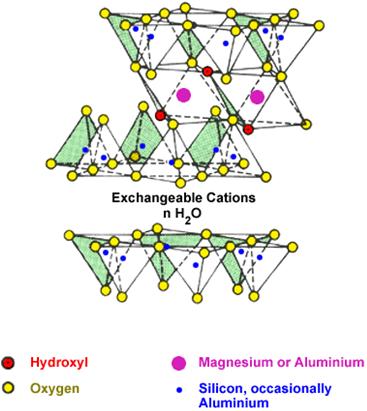
1.6. Montmorillonite Clay
Montmorillonite (MMT), the commonly used layered silicates for the preparation of polymer/layered silicate (PLS) nanocomposites belong to the same general family of 2:1 layered- or phyllosilicates (Alexandre & Dubois, 2000). Montmorillonite was first described in 1847 for an occurrence in Montmorillon in the department of Vienne, France, more than 50 years before the discovery of bentonite in the US.
Since MMT is environmentally friendly, naturally abundant and economic, it has been applied in numerous industrial fields due to its good performance-cost ratio. The outstanding feature of MMT is that the silicate layers can be expanded and even delaminated by organic molecules under proper conditions Thus, during the processing of polymer/MMT nanocomposites, the nanoscale silicate layers can be dispersed in the polymer matrix and the reinforcement phase forms in situ on the molecular level, which is very different from conventional filled composites. Moreover, it has been found that the polymer/MMT nanocomposites can be prepared by conventional processing techniques, such as extrusion and injection methods. Therefore, fabricating such nanocomposites is efficient and cost effective, which has drawn increasing attention in recent years, since montmorillonite reinforced nylon nanocomposites with excellent mechanic properties were developed by Toyota group.
2. Enhancement of Mechanical and Thermal Properties
Mechanical and thermal properties like tensile strength, elongation at break, modulus at 300% elongation, specific gravity and hardness, swelling index, flame retardency (FR) and abrasion resistance index (ARI) were increased in nano CaCO3 filled SBR than fly ash filled SBR [1].
2.1. Tensile Strength
Ts of nano CaCO3 filled SBR (2.58 Mpa for 9nm size CaCO3) are higher than commercial CaCO3 (1.63 Mpa) and fly ash filled SBR (1.37 Mpa).
2.2. Elongationat Break
Elongation at break of nano CaCO3 filled SBR (663% at 0.4 wt% of 9nm CaCO3) is higher than that of commercial CaCO3 filled SBR (345% at 0.4 wt%) and flyash filled SBR (360% at 40 wt%). This higher increment in elongation at break of nano CaCO3 is due to uniform dispersion of nano particles in rubber matrix.
2.3. Modulus at 300% Elongation
Maximum increment in modulus at 300% elongation is observed up to 0.4 wt% loading of nano CaCo3 and commercial CaCO3 and decreases above 0.4 wt% of nano CaCO3 filled SBR due to agglomeration of nano particles of filler. the moduli at 300% elongation are 1.2 Mpa for 0.4 wt% 9nm CaCO3 filled SBR, 0.62 Mpa for 0.4 wt% commercial CaCO3 filled SBR, while for 40 wt% of fly ash filled SBR the modulus at 300% elongation is 0.78Mpa.
2.4. Specific Gravity and Hardness
The increment in specific gravity and hardness is more appreciable in case of 9nm CaCO3 (1.35 at 0.5 wt%), commercial CaCO3(1.13 at 0.5 wt%), fly ash (1.18 at 50 wt%) filled SBR and pure SBR (0.9). The increase in specific gravity and hardness is due to greater and uniform dispersion of nano filler in matrix.
2.5. Swelling Index (SI)
At o.5 wt% of filler loading, SIsis 2.48 and 2.98 respectively for 9nm size CaCO3 and commercial CaCO3 filled in SBR. For fly ash filled SBR, SI is 2.90 at 50 wt% of filler loading. Thus in case of nano CaCO3, SI is least in comparison to other fillers due to greater cross linking of SBR, and uniform dispersion of nano CaCO3 in matrix.
2.6. Flame Retardancy
The flammability values are 1.97 and 1, 74 sec/mm respectively for 0.5 wt% of 9nm size CaCO3 and commercial CaCO3 whereas 50 wt% fly ash filled SBR shows 1.72 sec/mm rate. This increment in flame retardency is due to the nano fillers form effective layer on the surface, which absorbs the heat of burning.
2.7. Abrasion Resistance Index (ARI)
ARIs are 0.27g and 0.37g for 0.5 wt% of 9nm size CaCO3 and commercial CaCO3 respectively, while 50 wt% fly ash filled SBR shows ARI as 0.39g. This decrement in ARI is due to uniform dispersion of nano filler in rubber matrix.
When 9nm size CaCO3 is added with SBR, there was an increase in mechanical and thermal properties than that of commercial CaCO3 filled SBR and fly ash filled SBR.

 (S. Dwarakesh)
(S. Dwarakesh)  (G. S. Kumar)
(G. S. Kumar)