Captured Proteins by Immobilized Ricinus Communis Agglutinin in Rabbit Plasma
Manhua Liu, Xueke Zhou, Tingting Wang, Renqiang Li*
Department of Biotechnology, Jinan University, Guangzhou, China
Email address

(Renqiang Li)
*Corresponding author
Citation
Manhua Liu, Xueke Zhou, Tingting, Wang, Renqiang Li. Captured Proteins by Immobilized Ricinus Communis Agglutinin in Rabbit Plasma. AASCIT Journal of Bioscience. Vol. 2, No. 3, 2016, pp. 30-33.
Abstract
The Sepharose CL-6B column was prepared to separate ricinus communis agglutinin (RCA) from the crude extract of castor beans due to the gel carrier contains a large amount of β-galactoside bond. RCA were immobilized on activated Sepharose 4B to make an immobilized RCA column, which was used to capture the proteins in rabbit plasma by affinity chromatography. Results showed that two polypeptides with molecular mass about 120 and 97 KDa were captured by RCA in rabbit plasma. The captured polypeptides were demonstrated to contain the carbohydrate. The method performed in this study is much useful in the separation of sugary proteins.
Keywords
Sepharose CL-6B, Immobilized RCA, Captured Proteins by RCA, Affinity Chromatography, Plasma
1. Introduction
Plasma with the blood cells suspended has a crucial physiological role in the organism. There is a lot of proteins in the plasma, including many glycoproteins [1], which have many important functions including maintain plasma colloid osmotic pressure, composition of blood buffer system, involved in the maintenance of blood acid-base balance, transport nutrients and metabolites, nutritional function, involved in blood clotting and immune function and so on. Obviously, separation or purification of those glycoproteins in plasma is favor of a specific study of biochemistry of proteins.
Ricinus communis agglutinin (RCA) is a plant lectin produced from seeds of castor plant with a molecular mass about 130 KDa, is a tetramer composed of two A-type and two B-type chains [2,3]. RCA was of special interest because of its high affinity and particular specificity for glycoconjugates containing terminal β-D-galactoside bond [4-6], which explained that the RCA could be selected as the ligands to capture those glycoproteins in plasma by affinity chromatography. Therefore, the aim of this study is to probe or establish an available method for quickly and effectively separating those associated glycoproteins in plasma by affinity chromatography.
For preparation of this affinity chromatography, RCA was first separated from castor seeds by the aother affinity chromatography, Sepharose CL-6B was used due to these gel carrier contains a large amount of β-galactoside bond originally [8]. Then an immobilized RCA column was made and was used to separate the associated proteins in the plasma.
2. Materials and Methods
2.1. Materials
New Zealand white rabbits from Jinan University Experimental Animal Center, of which the plasma and red blood cells were prepared. The castor bean was from local market. Sepharose CL-6B and Sepharose 4B were from Amersham. Epichlorohydrin, 1, 4-dioxane and all other chemicals used in SDS-PAGE or for buffers used in the column chromatography were of analytical or chemical grade and were purchased from Guangzhou Chemical Co. Ltd. (Guangzhou, China). Markers for SDS-PAGE were supplied by Shanghai Sheng Zheng Biotechnology Co. Ltd. (Shanghai, China).
2.2. Extraction and Purification of the RCA
The preparation of RCA crude extraction was carried out according to the method of Niecolson [9]. The castor beans were bled in phosphate buffer (0.01 mol/L, pH 7.2, containing 0.15 mol/L NaCl) using the blender, the homogenate was slowly stirred at room temperature for 30 min, and followed at 4℃ overnight extraction. The sample was centrifugated at 10000 rpm for 30 min, repeated twice and the supernatant was obtained, which was salted out with 60% saturation of ammonium sulfate, the precipitate was dissolved in a small amount of phosphate buffer (0.01 mol/L, pH 7.2), and then dialyzed against the same buffer.
Due to the Sepharose CL-6B gel carrier contains a large amount of β-galactoside bond, which can be used as the ligand of RCA, so the Sepharose CL-6B affinity chromatography column was prepared to purify the RCA from the crude extract of castor bean. A column of Sepharose CL-6B with 1.5 cm × 10 cm was made and washed fully with equilibrium solution phosphate buffer (0.01 mol/L, pH 7.2, containing 0.15 mol/L NaCl), then 12 mL of RCA crude extract was applied to the column at a flow rate of 0.3 mL per min. After being washed fully with equilibrium solution, eluting solution (equilibrium solution containing 0.20 mol/L of galactose) was applied to elute the column. The chromatography process was under real-time monitored and a number of fractions at penetration peak and eluting peak were collected respectively and were concentrated by vacuum cold drying apparatus (Heto, High Technology of Scandinavia, Denmark), and dialyzed against deionized water before mixing with 1% SDS-mercaptoethanol solution as the SDS-PAGE sample.
Gel concentration used in SDS-PAGE was 12%. Analysis of SDS-PAGE pattern was performed using Electrophoresis Image Analysis System (FR-980, Furi Company, Shanghai, China).
2.3. Preparation of Immobilized RCA Column
2.3.1. Activation of Sepharose 4B
After the Sepharose 4B beads were washed using 1 mol/L NaCl and deionized water respectively at Buchner's funnel, 10 grams of these polymer beads were mixed with 6.5 mL 2 mol/L NaOH, 1.5 mL epichlorohydrin and 15 mL 56% 1, 4-dioxane, shaken for 2 h at 40°C for the activation of Sepharose 4B, and then were saturated in 0.1 mol/L, pH 9.5 Na2CO3-NaHCO3 buffer after being washed at funnel by using deionized water [10, 11].
2.3.2. Combination of RCA with Sepharose 4B
The activated Sepharose 4B beads were mixed with 10 mL of RCA dissolved in the 0.1 mol/L, pH 9.5 Na2CO3-NaHCO3 buffer, shaken at 40°C for about 24 h and washed at funnel using deionized water to collect the RCA that were not attached to the polymer beads. BCA kit was used to measure the RCA before and after mixed with the polymer beads. A column with 1.5 cm x 10 cm was made using these Sepharose 4B beads and for comparison, a control column of Sepharose 4B beads without RCA was also made.
2.4. Plasma Samples Loaded and Eluted
The immobilized RCA column was enough washed with equilibrium solution 0.02 mol/L, pH 7.4, Tris-HCl buffer, then 10 mL of rabbit plasma diluted ten-folds were loaded to the column at a flow rate of 0.3 mL per min. After being washed fully with equilibrium solution, eluting solution (0.02 mol/L, pH 4.0, NaAc-HAc buffer containing 0.15 mol/L NaCl) was applied to elute the column. The same performance was done on the control column. The chromatography process was under real-time monitored and a number of fractions at penetration peak and eluting peak were collected respectively, also freeze dried and detected by SDS-PAGE.
3. Results
3.1. Extraction and Purification of the RCA
The chromatography process of RCA crude extraction from Sepharose CL-6B column was shown in Figure 1. SDS-PAGE analysis of those proteins captured or uncaptured by β-galactoside bond in the Sepharose CL-6B column was shown in Figure 2.
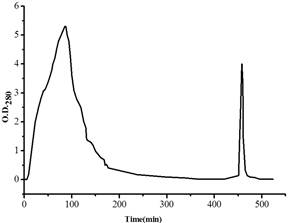
Figure 1. Chromatography process of RCA crude extraction on the Sepharose CL-6B column.
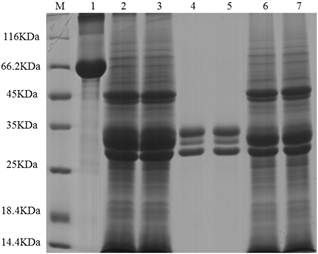
Figure 2. SDS-PAGE pattern of RCA from the Sepharose CL-6B column. M: protein markers. Lane 1: bovine serum albumin (BSA). Lane 2 and 3: RCA crude extract. Lane 4 and 5: elution peak of the column. Lane 6 and 7: penetration peak of the column.
Big peak refers to those proteins haven’t bound to the Sepharose CL-6B column, the second peak to the proteins captured by the column. The Sepharose CL-6B column was equilibrated with equilibrium solution phosphate buffer (0.01 mol/L, pH 7.2, containing 0.15 mol/L NaCl), and eluted with eluting solution (equilibrium solution containing 0.20 mol/L of galactose). The flow rate was about 0.3 mL per min, and the experiments were performed at room temperature.
The chromatography process of RCA by the Sepharose CL-6B column was very effective, not only in large numbers but also the pure RCA were obtained, which reflected on SDS-PAGE pattern. RCA is a tetramer composed of two A-type and two B-type chains with about 130 KDa molecular mass, of which the subunits are almost the same in 30-35 KDa. According to the comparison with those proteins in crude extraction and their molecular masses, the captured proteins by the Sepharose CL-6B column were judged as pure RCA. Further examination of agglutination of red blood cells (data not shown) demonstrated that the RCA sample possessed the activity, which explained too that the RCA had been obtained.
3.2. Proteins Captured by RCA
In the immobilized RCA column, the coupling rate 1.725 mg RCA fixed by gram of wet Sepharose 4B beads was obtained, so the column was used to practice. The chromatography process of rabbit plasma through the immobilized RCA column was shown in Figure 3. Some proteins were captured by RCA and their SDS-PAGE analysis was shown in Figure 4. In control column, not any elution peak was appeared (date not shown).
Big peak refers to those proteins haven’t bound to RCA, the second peak to the proteins captured by RCA. The RCA-Sepharose 4B column was equilibrated with equilibrium solution 0.02 mol/L, pH 7.4, Tris-HCl buffer, and eluted with eluting solution 0.02 mol/L, pH 4, NaAc-HAc buffer containing 0.15 mol/L NaCl. The flow rate was about 0.3 mL per min, and the experiments were performed at room temperature.
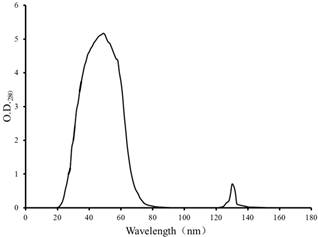
Figure 3. Elution profile of RCA-binding protein on RCA-Sepharose 4B affinity chromatography column.
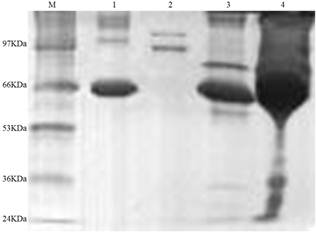
Fig. 4. SDS-PAGE pattern of rabbit plasma proteins from the immobilized RCA column.
M: protein markers. Lane 1: bovine serum albumin (BSA). Lane 2: elution peak of RCA-Sepharose 4B column. Lane 3: penetration peaks of RCA-Sepharose 4B column. Lane 4: rabbit plasma.
Athough the captured proteins in rabbit plasma by RCA were not so much (the second peak in Figure 3), two kinds of polypeptides captured by the RCA were appeared with molecular mass about 120 and 97 KDa (Lane 2 in Figure 4), respectively. In order to further verify the captured proteins, the UV absorption spectrum of mixture of RCA and the captured proteins in vitro was examined and shown in Figure 5. Captured proteins and RCA showed their protein absorption character at near 280 nm respectively, but after they were mixed together, this character of absorption spectrum was almost disappeared, which explained that RCA could react with the captured proteins, it was RCA that pull-down those proteins. For understanding if the carbohydrate was attached to the captured proteins, the colorimetric phenol-sulfuric acid method [12,13] was used to examine the samples. Results of O. D.490 of the control, RCA, captured proteins and their mixture were 0, 0.141, 0.195 and 0.310, respectively, which explained that the carbohydrate were attached to both RCA and captured proteins, the latter may be the glycoprotein.
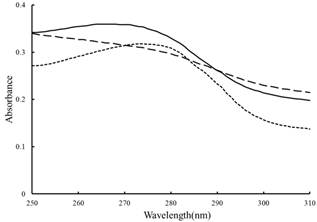
Fig. 5. UV absorption spectra of RCA and the proteins captured by RCA before and after their mixture.
Dotted line: RCA. Solid line: the proteins captured by RCA. Dashed line: the mixture of RCA and the proteins captured by RCA.
4. Discussion
Due to that a lot of glycoproteins are existed in plasma, many of which are still unknown clearly for their important physiological functions, to establish an available method for quickly and effectively separating the glycoproteins is deserved. Many studies have been performed on the RCA, here RCA were only used as the ligands to separate the proteins in plasma due to their special reactions with sugary proteins. As expected, some proteins were captured by RCA in rabbit plasma with two polypeptides were obtained in this study, which demonstrated that the aim of this study has been realized. Obviously, the method performed in this study is much useful in the separation of sugary proteins because of a lot of existence of biological samples containing sugary proteins.
For further demonstration that those captured proteins were pull-down by RCA, the UV absorption spectra of RCA and those proteins captured by RCA before and after their mixture in vitro were examined. After mixed together, a great change of the absorption character at near 280 nm of protein was happened, which could explain that the captured proteins were really pull-down by RCA, and also explained the complexity of reactions between RCA and their binding proteins.
Because the captured proteins by RCA were a mixture with two polypeptides, it was difficult to determine if every captured polypeptide by RCA was the glycoprotein, although the captured proteins mixture was demonstrated to contain the carbohydrate. It was especially difficult to determine the type of carbohydrate attached to the captured protein. The RCA obtained from Sepharose CL-6B column was examined to contain the carbohydrate too, which may be due to the use of galactose during the eluting of RCA from the Sepharose CL-6B column. Also due to the captured proteins by RCA was a mixture, it made us harder for verifying the type of every polypeptide, which would be our further work in future.
5. Conclusions
The first affinity chromatography was used to separate RCA for establishing the immobilized RCA column, the second affinity chromatography of the immobilized RCA column was used to capture the glycoproteins in rabbit plasma, of which two polypeptides with molecular mass about 120 and 97 KDa were obtained. The method performed in this study is much useful in the separation of sugary proteins from biological samples.
References
- Schwick, HG., Haupt, H., (1981), Purified human plasma proteins of unknown function. Jap. J. Med. Sci. Biol., 34: 299-327.
- Liener, IE., (1986), The lectins: Properties, Functions, and Applications in Biology and Medicine, Academic Press, San Diego, CA, and London.
- Rutenber, E., Robertus, JD., (1991), Structure of ricin B-chain at 2.5A resolution. Proteins. 10: 260-269.
- Wang, Y., Yu, G., Han, Z., Yang, B., Hu, Y., Zhao, X., Wu, J., Lv, Y., Chai, W., (2011), Specificities of Ricinus communis agglutinin 120 interaction with sulfated galactose. FEBS Letters, 585: 3927-3934.
- Wu, J., Herp, A., Wu, A., (1993), Defining carbohydrate specificity of Ricinus communis agglutinin as Gal beta 1-->4GlcNAc (II) > Gal beta 1-->3GlcNAc (I) > Gal alpha 1-->3Gal (B) > Gal beta 1-->3GalNAc (T). Molecular Immunology, 30: 333-339.
- Wu, A., Wu, J., Singh, T., Lai, L., Yang, Z., Herp, A., (2006), Recognition factors of Ricinus communis agglutinin 1 (RCA(1)). Molecular Immunology, 43: 1700-1715.
- Zhou SM,Cheng L,Guo SJ,Wang Y,Czajkowsky DM,Gao H,Hu XF,Tao SC., (2015), Lectin RCA-I specifically binds to metastasis-associated cell surface glycans in triple negative breast cancer.Breast Cancer Res, 17(1): 544-558.
- De-Simone, SG., Netto, CC., Silva Jr., EP., (2006), Simple affinity chromatographic procedure to purify beta-galactoside binding lectins. J. Chromatogr. B, 838: 135-138.
- Nicolson, GL., Blaustein, J., (1972), The interaction of Ricinus communis agglutinin with normal and tumor cell surfaces. Biochim Biophys Acta, 266(2): 543-547.
- Li, R., Jiang, F., Zhang, X., Chen, Y., Fang, L., (2006), Immobilized hemin affinity chromatography as a probe for proteins having potentiality to bind with heme. J. Chromatogr. B, 840: 63-68.
- Cai, H., Xie, Y., Hu, L., Fan, J., Li, R., (2013), Prion protein (PrP(c)) interacts with histone H3 confirmed by affinity chromatography. J Chromatogr B, 929: 40-44.
- Saha, SK., Brewer, CF., (1994), Determination of the concentrations of oligosaccharides, complex type carbohydrates, and glycoproteins using the phenol-sulfuric acid method. Carbohydr Res., 254: 157-167.
- Dong, Q., Zheng, L., Fang, J., (1996), Modified phenol-sulfuric acid method for determination of the content of oligo-and polysaccharides. Chin Pharm J., 31: 550-553.

 (Renqiang Li)
(Renqiang Li) 






