Induction of Haematological and Lipid Profile Changes by Aluminium-Induced Toxicity and Ameliorative Effects of Selected Antioxidants on Wistar Rats
Anacletus F. C., Onyegeme-Okerenta B. M.*
Department of Biochemistry, Faculty of Science, University of Port Harcourt, Choba, Rivers State, Nigeria
Email address

(Onyegeme-Okerenta B. M.)

(Onyegeme-Okerenta B. M.)
*Corresponding author
Citation
Anacletus F. C., Onyegeme-Okerenta B. M. Induction of Haematological and Lipid Profile Changes by Aluminium-Induced Toxicity and Ameliorative Effects of Selected Antioxidants on Wistar Rats. Health Sciences Research. Vol. 3, No. 3, 2016, pp. 30-34.
Abstract
This study evaluated the changes on haematology indices and lipid profile of aluminium-induced toxicity and ameliorative effects of selected antioxidants on wistar rats. Ninety-six wistar rats (48 males and 48 females) were used and grouped into eight groups (control, aluminium, zinc, selenium, ginseng, vitamin A, vitamin C and vitamin E) and were allowed to acclimatize in their new environment for 21days. Administration of the aluminium and antioxidants started immediately. Group 1 was the control and no chemical was administered, group 2 was administered 200mg/kg of aluminium alone while groups 3-8 were administered zinc, selenium, ginseng, vitamin A, vitamin C and vitamin E alongside aluminium with dosages of 14.8mg/kg, 100mg/kg, 10mg/kg, 100mg/kg, 100mg/kg,100mg/kg and 200mg/kg respectively. The animals were exposed to treatments once daily for six weeks after which the animals were sacrificed and blood samples were collected and analyzed in the laboratory. Results obtained showed significant increase (p<0.05) in the concentration of cholesterol, triglycerides, HDL and LDL in the aluminium group when compared with that of the control group. The various antioxidants however significantly (p<0.05) ameliorated the effect of the aluminium toxicity by reducing the various concentration of cholesterol, triglycerides, LDL and increasing that of HDL. Similarly, there was significant decrease (p<0.05) in the haematological indices indicating thrombocytopenia, anaemia and leucopenia. However, co-administration of aluminium and antioxidants especially vitamins C and E resulted in significant increase (p<0.05) in the haematological indices.
Keywords
Aluminium, Toxicity, Haematology, Lipid, Antioxidants
1. Introduction
Aluminium is a trivalent cation found in its ionic form in most kinds of animal and plant tissues and in natural waters everywhere. It is a ubiquitous element and the third most prevalent (abundant) element in the earth's crust, comprising approximately 8% of the earth’s crust, exceeded only by oxygen (47%) and silicon (28%). The presence of this element has so heavily contaminated the environment that exposure to it is virtually inescapable. Natural processes account for most of the redistribution of aluminium in the environment [1] as a result of the weathering of rocks and minerals in which it is present. Mobilization from natural sources can, however, also result from the deposition of acidic precipitation [2]. Direct anthropogenic releases of aluminium compounds occur primarily to air and these are associated with industrial processes. Thus, the mining and processing of aluminium ores and the production of aluminium metal, alloys and compounds can lead to the release of aluminium compounds into the environment. The use of aluminium and its compounds in processing, packaging and storage of food products, and as flocculants in the treatment of drinking-water may contribute to its presence in drinking water and food stuffs [1].
Daily intake by humans is estimated to be 1-10 mg (0.04 - 0.37 mmols). It is difficult to provide generalized estimates of environmental concentrations because of the complexity of its chemistry which is controlled by pH, mineralogical constituents and the quantities and indeed quality of associated natural organic matter. Aluminium from use of deodorants caused contact dermatitis to the skin. The health effects of antiperspirants are a matter of dispute regarding their extent. A small percentage of people are allergic to aluminium and may experience contact dermatitis when exposed to aluminium-containing deodorants. A study done by the University of Cincinnati Medical Center showed that using aluminum pots and pans to cook tomatoes doubled the aluminum content of the tomatoes, from 2 to 4 milligrams per serving [3]. Aluminium is one of the few abundant elements that appear to have no beneficial function to Living cells [4]. At high doses, Aluminium itself adversely affects the blood-brain barrier, is capable of causing DNA damage, and has adverse epigenetic effects [5]. The use of aluminium-containing antiperspirants has been linked with the systemic accumulation of aluminium which increases the risk of Alzheimer's disease [6]. Aluminium also has a direct effect on hematopoiesis. Excess aluminium has been shown to induce microcytic anaemia. Daily injections of aluminium into rabbits produced severe anaemia within 2-3 weeks. Aluminium may cause anemia through decreased heme synthesis, decreased globulin synthesis, and increased hemolysis. Aluminum may also have a direct effect on iron metabolism: it influences absorption of iron via the intestine, it hinders iron's transport in the serum, and it displaces iron's binding to transferrin. Patients with anaemia from aluminium toxicity often have increased reticulocyte counts, decreased mean corpuscular volume, and mean corpuscular haemoglobin [4], [7], [8].
Many patients with renal failure and haemodialysis have anaemia caused by high doses of hydroxyl aluminium gel over long periods to control serum phosphorus levels; the majority of aluminium found in serum is bound to tissue factor, which is responsible for the transport of iron. Tissue factor and iron are crucial regulators of erythropoiesis; thus an immediate suggestion of mechanism behind anaemia in patients afflicted with renal failure is evident that implicates aluminium [9].
Despite the fact that Aluminum is a non-redox metal, it has been reported that mice chronically fed with this metal presented high levels of brain tissue lipid peroxidation [10].The end products of lipid peroxidation are cytotoxic and may cause platelet dysfunction. Nevertheless, reactive oxygen species (ROS) are generated by platelets [11]. ROS are central to a host of pathologies, including inflammation, toxicity, and endocrine disruption by environmental chemicals and are degraded by the organized system of antioxidants. Antioxidants have been described as substances that either directly or indirectly protects cells against adverse effects of xenobiotics, carcinogens, drugs and toxic agents. Aluminium can incite oxidation of molecule in the body thereby resulting in oxidative stress; the present study therefore evaluates the haematology indices and lipid profile of aluminium-induced toxicity and ameliorative effects of selected antioxidants on wistar rats.
2. Experimental Design
A total of 96 wistar rats were obtained from the animal house of the department of animal and environmental biotechnology, University of Port Harcourt, Rivers State, Nigeria. They were housed in separate plastic cages and acclimatized for twenty-one days and fed on conventional rat feed and water. The rats were completely randomized into eight groups of six rats each; (control, aluminium, selenium, zinc, ginseng, vitamin A, vitamin C and vitamin E). Rats in the control group were given only their food and distilled water, the aluminium group was given 200mg/kg of aluminium alongside their food. The other groups which include the selenium, zinc, ginseng, vitamin A, vitamin C and vitamin E were given 200mg/kg of aluminium as well as 100mg/kg, 14.8mg/kg, 10mglkg, 100mg/kg, 100mng/kg and 100mg/kg of the various dosages of antioxidants respectively. At the end of six weeks of administration, two wistar rats from each group were selected and sacrificed by anaesthetizing with chloroform and their blood taken to laboratory for analysis.
3. Methodology
3.1. Collection of Blood Samples
Blood samples were collected by cardiac puncture and allowed to clot for 2 hours at room temperature, followed by centrifugation at 3500 g for 10 minutes to obtain the serum.
3.2. Determination of Lipid Profile
Total Cholesterol, triglycerides, LDL and HDL were analyzed by kinetic methods kits from Randox, (United Kingdom) using a double-beam spectrophotometer.
3.3. Determination of Haematological Parameters
Haematological parameters (haemoglobin concentration, packed cell volume, total white blood cell count, platelet count, neutrophils and lymphocytes) were determined using the methods as described by [12].
3.4. Statistical Analysis
In this study, data was analysed by one way ANOVA according to SPSS, version 21 program to find the means for all treatments. These means were compared using Turkey HSD and Bonferroni at 0.05 confidence limit (p< 0.05) in multiple comparison.
4. Results
4.1. Effect of Aluminium and Antioxidants on Haematology Indices
Figure 1 shows the effect of aluminium and antioxidants on haematology indices. There was significant decrease (p<0.05) in haemoglobin level in the group administered with only aluminium. Significant increase (p<0.05) was observed in the groups administered with aluminium + Zinc, Selenium, ginseng, vitamin A, C and vitamin E when compared with the group treated with aluminium only.
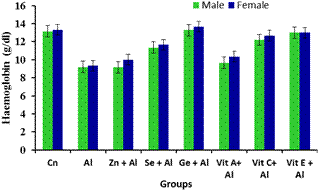
Figure 1. Effect of Aluminium and selected anti-oxidants on Haemoglobin concentration of male and female rats.
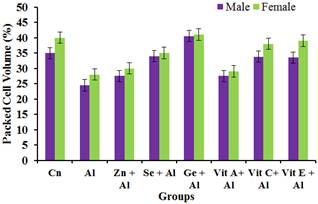
Figure 2. Effect of Aluminium and selected anti-oxidants on Packed Cell Volume of male and female rats.
Figure 2 showed a significant decrease (p<0.05) in the packed cell volume of the group treated with aluminium. On the other hand, there was significant increase (p<0.05) in the groups treated with aluminium + Zinc, Selenium, ginseng, vitamin A, C and vitamin E when compared with the group treated with aluminium only.
From Figure 3, a significant decrease (p<0.05) in the white blood cell count of the aluminium group was observed. While there was significant increase (p<0.05) in the white blood cell count in groups treated with aluminium + zinc, ginseng, vitamin A & vitamin C groups, there was no significant increase (p>0.05) in groups treated with aluminium + selenium and vitamin E when compared with the group treated with aluminium only.
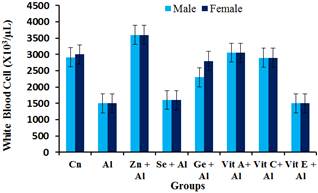
Figure 3. Effect of Aluminium and selected anti-oxidants on White Blood Cell of male and female rats.
Figure 4 shows the effect of aluminium on neutrophils. A significant decrease (p<0.05) was observed in the aluminium treated group while there was no significant decrease (p<0.05) in the groups treated with aluminium and the antioxidants.
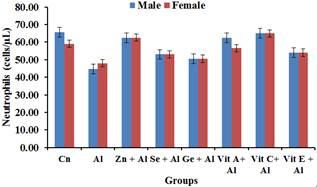
Figure 4. Effect of Aluminium and selected anti-oxidants on Neutrophils of male and female rats.
Figure 5 shows a significant increase (p<0.05) in the aluminium treated group. There was no significant increase (p<0.05) in the lymphocytes level of rats fed with aluminium and the antioxidants when compared with groups treated with aluminium only.
Figure 6 shows a significant decrease (p<0.05) in the platelets value of the groups treated with only aluminium. However, significant increase (p<0.05) was observed in the groups treated with aluminium and the antioxidants when compared with groups treated with aluminium only.
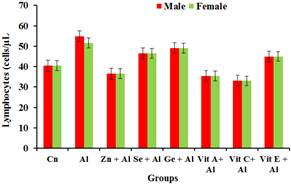
Figure 5. Effect of Aluminium and selected anti-oxidants on lymphocytes of male and female rats.
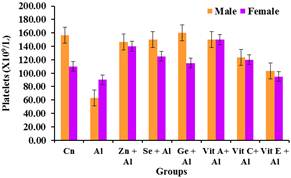
Figure 6. Effect of Aluminium and selected anti-oxidants on Platelets of male and female rats.
4.2. Effect of Aluminium and Antioxidants on Lipid Profile
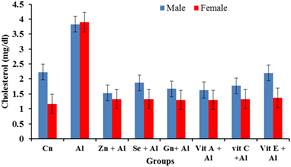
Figure 7. Effects of aluminium and antioxidants on the cholesterol concentration of male and female rats.
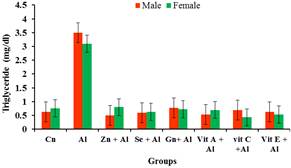
Figure 8. Effects of aluminium and antioxidants on the triglycerides concentration of male and female rats.
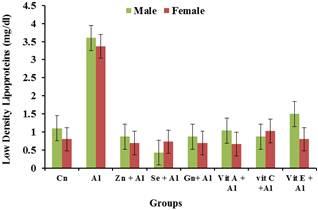
Figure 9. Effects of aluminium and antioxidants on the LDL concentration of male and female rats.
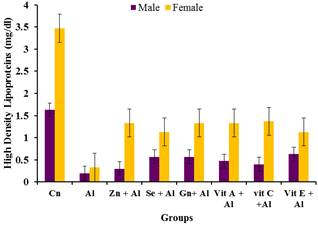
Figure 10. Effects of aluminium and antioxidants on the HDL concentration of male and female rats.
5. Discussion and Conclusion
The prevalence of cardiovascular disease (CVD) related deaths in the world are on the increase and high blood pressure is one of the major contributors of these diseases. The clinical consequences of these conditions are severe and exert major research efforts to improve knowledge of its pathogenesis and thereby provide a more rationed approach to its prophylaxis and therapy [13]. From the result obtained, it was observed that there was significant increase (p<0.05) in the levels of cholesterol, LDL and triglycerides in the serum of the aluminium treated rats compared to the control groups while the HDL was significantly reduced (p<0.05) in the aluminium treated group when compared to the control group. This may indicate aluminium toxicity. However the various antioxidants were able to significantly (p<0.05) ameliorate the effects of the aluminium toxicity. These findings agree with [14] who reported the effects of aluminum sulphate treated in deionizable and tap water on lipid profile of wister rats. Their findings showed that there was increase in lipid profile with increasing dose of Aluminum sulphate. Similarly, Triglycerides and total cholesterol levels increase in response to aluminum toxicity observed in this study is consistent with increasing lipogenesis in the liver as observed by [15]. Amelioration by the various antioxidants were significantly pronounced in the female rats than in the male rats when compared to that male and female groups treated with aluminium.
Results obtained from study of haematology indices showed that there was significant (p<0.05) differences in the full blood count induced by aluminium. A significant decrease (p>0.05) in haemoglobin, PCV, WBC, and platelets was observed in rats treated with aluminium. Aluminium toxicity induced anaemia, leucopenia and thrombocytopenia. These findings were consistent with [16], who reported a decrease in haemoglobin, red blood cells, as well as the related markers in human in chronic exposure to aluminium citrate. However, the antioxidants zinc, selenium, ginseng, Vitamin A, C and E administered significantly ameliorated the toxic effect of aluminium on haemoglobin and other haematology indices when compared with the aluminium treated group. The effect of Vitamin C and E was also observed by [17], where the action of Vitamin C as an anti-oxidant prevented the binding of free radicals on DNA by activation of detoxification and protection of capillary walls.
These findings suggests that Aluminium which is ubiquitous in our environment and whose importance in our daily lives cannot be overemphasized causes toxicity when accumulated in various organs in the body and the blood cause different forms of damages to these organs. It can be concluded that aluminium toxicity increases the concentration of cholesterol and other lipid parameter in the body and hence result in cardio vascular disease as well as anaemia, leucopenia and thrombocytopenia. However the various antioxidants were able to ameliorate the effect of aluminium toxicity by reducing cholesterol, Triglycerides, LDL and increasing HDL. It also had positive effects on the haematology indices. Therefore the consumption of these antioxidants may be recommended for use as dietary supplements to rid the body of toxicity caused by heavy metals like aluminium.
References
- ATSDR (Agency for Toxic Substances and Disease Registry): (2005). "Toxicological profile for aluminum and compounds". Atlanta, GA.: U.S Department of Health and Human Services, Public health service.
- Wagner, W. (1999). "Canadian Minerals Year book". Ottawa: Natural Resources Canada.
- Abreo, V. (2015). The Dangers of Aluminium Toxicity.BellaOnline's Alternative Medicine Editor. http://www.bellaonline.com/articles/art12624.asp. http:// www.bellaonline. Com/articles/ art7739.asp.
- Exley, C., Charles, L. M., Barr, L., Martin, C., Polwart, A. & Darbre, P. D. (2007). "Aluminium in human breast tissue". Journal of Inorganic Biochemistry101 (9):1344–6.
- Lankoff, A., Banasik, A. & Duma, A. (2006). "A comet assay study reveals that aluminium induces DNA damage and inhibits the repair of radiation-induced lesions in human peripheral blood lymphocytes". Toxicology Letters, 161 (1): 27–36.
- Graves, A. B., White, E., Koepsell, T. D., Reifler, B. V., van Belle, G. & Larson, E. B. (1990). "The association between Aluminium-containing products and Alzheimer's disease". Journal of Clinical Epidemiology 43 (1): 35–44.
- Mahieu, S., Contini, M., Gonzalez, M., Millen, N. & Elias, M. M. (2000). Aluminum toxicity. Hematological effects. Toxicology Letters, 111: 235-242.
- Zaman, K., Zaman, W. & Siddique, H. (1993). Hematological and enzymatic results of aluminum intoxication in rats. Comparative Biochemistry and Physiology, 105: 73-76.
- Eschbach, J. W., Varma, A., & Stivelman, J. C. (2002). Is it time for a paradigm shift? Is erythropoietin deficiency still the main cause of renal anaemia? Nephrology Dialysis Transplantation, 17 (5): 2-7.
- Fraga, C. G., Oteiza, P. I., Golub, M. S., Gerswin, M. E. & Keen, C. L. (1990). Effects of aluminium on brain lipid peroxidation.Toxicology Letters, 51: 213-219.
- Salvemini, D. & Botting, R. (1993). Modulation of platelet function by free radicals and free radical scavengers. Trends in Pharmacological Sciences, 14: 36-42.
- Dacie, J. V. & Lewis, S. M. (2006). Investigation of haematological disorders. Practical Haematology, Churchill Livingstone Edinburgh, United Kingdom, pp: 177-180.
- Kritchersky, (1970). Role of cholesterol vehicle in experiment atherosclerosis. American Journal of Clinical Nutrition, 23: 1105-1110.
- Salah, E. I., Sabahelkhier, M. K. & Shama, I. Y. Adam. (2015). Effects of aluminum sulphate treated in deionizable and tap water on lipid profile of wister rats. ARPN Journal of Science and Technology, 5(5): 268-270.
- Thirunavukkarasu, C. & Sakthisekaran, D. (2003). Influence of sodium selenite on glycoprotein contents in normal and N-nitrosodiethylamine initiated and phenobarbital promoted rat liver tumors. Pharmacological Research 48:167-173.
- Samani, K. G., Farrokhi, E., Samani, N. M. & Moradi, H. H. (2015).The effect of Aluminum on the increasing risk of developing anemia among workers of tile production plants. International Journal of Epidemiologic Research, 2(1) 24-29.
- Siess, M. H., Le Bon, A. M., Canivenc-Lavier, M. C. & Susch, M. (2000). Mechanisms Involved in the Chemoprevention of Flavonoids, Biofactor, 12 (4), 193-199.

 (Onyegeme-Okerenta B. M.)
(Onyegeme-Okerenta B. M.)  (Onyegeme-Okerenta B. M.)
(Onyegeme-Okerenta B. M.) 











