Simultaneous Dyeing and Fragrance Finishing of Cotton Fabric
A. K. Samanta, Anowar Hossain, A. Bagchi, Kashmita Bhattacharya*
Department of Jute and Fibre Technology, Institute of Jute Technology, University of Calcutta 35 Ballygunge Circular Road, Kolkata, India
Email address

(A. K. Samanta)

(K. Bhattacharya)

(A. Hossain)
*Corresponding author
Citation
A. K. Samanta, Anowar Hossain, A. Bagchi, Kashmita Bhattacharya. Simultaneous Dyeing and Fragrance Finishing of Cotton Fabric. Journal of Materials Sciences and Applications. Vol. 2, No. 4, 2016, pp. 25-34.
Abstract
A newer method of simultaneous dyeing and finishing has been developed using pad-dry-cure method by using limited aqueous solution of reactive dye, citric acid and suitable fragrance chemical microencapsulated in beta-cyclodextrin solubilized in PEG 200 and water (1:3) along with Citric acid and Sodium hypophosphite as catalyst applied on cotton fabric by pad-dry-cure method. Spectrophotometer analysis of combined dyed and finished cotton fabric show a deeper color yield and good aroma finishing using monochlorotriazine reactive dye along with jasmine oil microencapsulated in beta cyclodextr in in presence of citric acid and sodium hypophosphite applied on cotton fabric. For comparison purpose, beta cyclodextrin encapsulated aroma finish was also applied after dyeing cotton fabric with the same monochlorotriazine on cotton fabric in conventional two stage process. Simultaneous reactive dyeing and aroma finishing with beta cyclodextrin and citric acid show yield of K/S value, dry crease recovery angle and better retention of tensile strength than the two stage conventional method. FTIR spectra confirm that crosslinking successfully formed among the carboxyl group of citric acids and hydroxyl groups of cellulose and beta cyclodextrin forming ester crosslinks. Jasmine oil was also applied on cotton with beta cyclodextrin by micro encapsulation technique and without beta cyclodextrin by normal pad-dry-cure method. Jasmine oil applied with beta cyclodextrin by microencapsulation show higher level of retention of fragrance intensity for simultaneous in combined dyeing and finishing technique than two stage conventional methods. Human sensory evaluation indicates that fragrance intensity became gradually weaker with respect to time and higher nos. of washing cycle. Fragrance intensity after different duration of normal stoppage and also after nos. of wash cycles was tested by gas chromatography and, the fragrance intensity of Jasmine was found to be 15.88% after five repeat washing and the fragrance intensity was retained 39.5% in the cotton fabric up to 100 days of normal storing without washing. This single bath simultaneous reactive dyeing and aroma finishing also offers advantages of reduction of process cost, saving in energy, reduction in treatment time as well as better color yield and therefore is of commercial importance too.
Keywords
1. Introduction
Simultaneous dyeing and finishing in a single bath offers considerable economy in operating costs because of the reduction in process steps, time, energy etc besides simplicity of the operations. So, an attempt was made in the present work to carry out simultaneous reactive dyeing and aroma finishing in a single step instead of two step conventional processes. Aroma finish on cotton fabric or garments render special value addition in terms of reducing stress, relaxation of minds by release of pleasant odor from textiles even helping nice sleep etc. Aroma finishing after reactive dyeing by conventional two stage process as reported in literature [1] but simultaneous reactive dyeing and aroma finishing of cotton in a single bath technique is a newer concept, attempted in the present work. The investigation [2] of US retail sales of home fragrance for pleasure, enjoyment and reducing stress shows that total sales increased to about 9-10%, in 4 yrs time i.e. from $1.35 billion in 1995 to $1.96 billion in 1999.The scents of lavender, rose, citrus or vanilla were micro encapsulated into cotton fabrics [3,31,32] with or without dyeing in a conventional method, which is proved to be a good approach to meet the customers demand of important psychological and emotional needs, as well as also for purely physical and sensorial nature-aromatherapy. This concept was first coined in the late 1920s by the French cosmetic chemist R.M. Gattefosse [4], who noticed the excellent antiseptic properties and skin permeability of some fragrance. Dextrinhas OH group and Cyclodextrins [3, 5] are cyclic oligosaccharides containing six to thirteen 1,4-linked glycopyranose units with hydrophilic hydroxyl groups on their outer surface creating also a hydrophobic cavity in the center capable of incorporating any hydrophobic or oleophilic material, like fragrance oil as well. D. kaur et al and other researcher [9-15] informed that the cyclodextrin has capacity to form inclusion of other hydrophobic materials to form a complex. J.-P. Moldenhauer [7] found that β-cyclodextrin is a reactive cyclodextrin derivative that can be covalently fixed to nucleophilic substrates by a substitution reaction. Textile used as Underwear, gloves, socks [5, 8] may be loaded with the necessary antifungal, antihistaminic, and anti-inflammatory agents by such microencapsulation. Similarly Flowers, leaves and fruit used as ingredients [16,17] of perfume a few a molecule [18] of which is sufficient for us to experience its odor or to identify the smell. There are many microencapsulation technique [19, 20, 21] including Complex Coacervation, Polymer-Polymer Incompatibility, Interfacial Polymerisation and In Situ Polymerisation, Spray Drying, Centrifugal Extrusion, Air Suspension Coating, Pan Coating, Emulsion Hardening Process and Co-precipitation method etc [27,30] β-cyclodextrin is said to free from skin irritation, no skin sensibilisation and any mutagenic effect.
β-cyclodextrinmolecules has cone-shaped hydrophobic [28] cavity where aroma oils are microencapsulated and as a result, the rate of release of fragrance are greatly reduce for a long period of storage.
Considering all the above said information, it is thought appropriate to undertake an attempt to develop a newer one bath simultaneous reactive dyeing and aroma finishing of cotton fabricfor special value addition in a economic approach.
2. Experimental
2.1. Materials
2.1.1. Cotton Fabric
Bleached Plain weave 100% cotton fabric was used as per following particulars. Fabric ends/decimeter: 13.75, pick/decimeter: 14.73, GSM: 54.
2.1.2. Chemicals and Dyes
Reactive dye of Dyechem international, Kolkata, Beta Cyclodextrin of TCI Chemical Ltd. India.
Dextrin, Polyethylene Glycol, Sodium hypophosphite, Sodium Carbonate, Sodium chloride, Citric acid of laboratory grade were used. Fragrance chemical (Jasmine) → LN chemical industries ltd, Mumbai, India.
Polyethylene Glycol-200 and Distilled water in ratio 1:3was used as solvent, Citric acid was used as crosslinking agent, Sodium hypophosphite was used as catalyst and Beta Cyclodextrin was used in different dosage like 5%, 10%& 15%. Fragrance chemical like Jasmine oil, usual dyeing auxiliaries (like NaCl, Na2CO3etc) for reactive dye. Beta cyclodextrin (95% purity) was used as microencapsulating agent. Reactive dye- Lemon Yellow-CN-1% obtained from Dyechem International, was used besides Jasmine oil -1%, 2%, 3% application was used for both simultaneous reactive dyeing and aroma finishing as well as for two stage dyeing and finishing process.
2.2. Methods
2.2.1. Preparation of Microencapsulation of Fragrance Chemical
A solubilized solution of beta cyclodextrin was prepared with the addition of polyethylene glycol and water (1:3) at 50°C. Fragrance Chemical was added in the solution drop by drop with continuous stirring for proper microencapsulation.
2.2.2. Method of Combined Dyeing and Fragrance Finishing
Reactive dye lemon yellow-CN (CI Reactive Lemon Yellow) -1%, Citric acid – 10%and Sodium hypophosphite-6% were added in the above microencapsulated solution containing beta cyclodextrin (5%, 10%, 15%) and synthetic fragrance-1%with stirring for dissolving. Bleached fabric was impregnated in above solution and padded with 80% expression by two dip two nip process. Then, the cotton fabric was dried at 100°C and Cured at 150°C for 5 min. Similarly Dextrin (5%, 10%, and 15%) was used for comparative study.
To study the role of beta cyclodextrin, experiments were also conducted by using synthetic fragrance (1%, 2%, 3%) with 10% beta cyclodextrin and without beta cyclodextrinto find out fragrance intensity as well as microencapsulation capability of beta cyclodextrin.
2.2.3. Method of Conventional Two Stage Dyeing and Finishing
First stage (Dyeing):
Bleached cotton fabric was first dyed with reactive dye lemon yellow CN-1% with 1:20 liquor ratio at 80°C and after 15 min required amount of sodium chloride and sodium carbonate was added two parts. After dyeing the sample was rinsed, soaped and dried.
Second Stage (Finishing): The above ready dyed fabric was finished with the same recipe (without dyestuff) and procedure of above combined dyeing and finishing.
2.2.4. Testing Methods
i) Color strength (K/S value) was measured by measuring surface reflectance value where Computer color measuring instrument (Macbeth 2020 plus spectrophotometer). Color strength of untreated and treated cotton fabric samples is measured from the reflectance value at maximum wave length from which surface color strength (K/S value) can be determined by kubelka- munk equation:
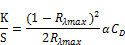
Where, K = coefficient of absorption.
S = coefficient of scattering.
Rλmax = reflectance of the substrate at maximum absorbance wavelength.
CD = concentration of dye.
ii) Color fastness to wash was tested by IS II method (i.e. ISO – 105-C-10, 2006 standard using 5 gpl soap, 2 gpl soda ash at 60°C for 30 mints, IS -3361-1984 method) with Launder-o-Meter. The wash cycles were repeated 1, 2, 3, 4, 5 times to examine the fragrance retention after respective nos. of wash cycles.
iii) Light fastness to wash was tested by AATCC TM16 in Q-SUN XE-2 Xenon Test chamber against blue wool standards (BS -1006-BOI – 1978).
iv) Dry crease recovery was measured by ASTM-D-1295-67 in Shirley crease recovery tester.
v) Tensile strength and elongation % were evaluated by Ravel strip method using 20 mm X 5 mm sample size after raveling as per IS-1969-1985 method using Instron machine.
vi) Fourier transform infrared spectra (FTIR) FTIR spectroscopic analysis of untreated and treated cotton fibre was palleted in spectroscopic grade KBr pallet and was subjected to generate FTIR spectra for respective samples using Parkin Elmer, UK. Model: Spectrum-100 FTIR spectrometer.
vii) Olfactometric analysis of odour retention (for retention of fragrance):
A Human sensory analysis by opinion pole of group of experts – Human sensory analysis to establish the presence of intensity of odour of Rose by Nasal sensory opinion of a group of expert panel was taken after each wash (1 to 5 cycles) and after 10 to 100 days of storing without wash. The odour concentration / intensity was subdivided into the following categories [27]:
0 – No Odour (0%)
1 – Very weak (Around 5 – 15%)
2- Weak (Around 20 – 40%)
3 – Medium strong (Around 50 – 70%)
4 – Strong (Around 75 – 85%)
5 – Very strong (Around 90 – 95%)
B Gas Chromatography Testing (Capillary method) for test of retention of Aroma oil(Rose oil) after different cycle of wash and different durations of storage:
Detail procedure of GC analysis is as follows:-
High purity gases are supplied to the GC. One of the gases (called the carrier gas) flows into the injector, through the column and then into the detector. All gases-injector carrier, detector gases are fully electronically controlled through advanced flow controller and advanced pressure controller. Firstly standard rose has been injected with a syringe or an exterior sampling device in gas chromatography machine and the volatile solution are transported into the column by the carrier gas where the column is maintained in a temperature controlled oven. Solution enters heated detector and an electronic signal is generated upon interaction of the solute with the detector. The size of the signal is recorded by a data system and is plotted against elapsed time to produce a chromatogram and peak of fragrance has been recorded automatically by detector software crom card Fragrance treated fabric has been extracted by acetone. Then extracted solution has been injected and expected peak of sample/fragrance treated fabric has been found comparing recorded peak of standard rose for quantitative analysis of rose oil quantity still present after different duration of storage or after different nos. of wash cycles / different days of storing.
Testing was done by using Column: TG 5 MS, Capillary Column, Column Temperature 100°C, injected temperature 190°C, Detector temperature 220°C, Nitrogen carrier gas flow 1.5 mi /min, speed ratio 1:33, Software detector: FID (Flame ionization detector) and software crom card using a Gas Chromatography Instrument make: Thermo fisher, Trace GC 1110, India.
3. Result and Discussion
3.1. Study on Dyeing Properties of Dyed and Finished Cotton Fabric
First of all, dyeing property of combined dyeing fragrance finishing as well as conventional two stage process has been studied. The changes in dyeing property of bleached cotton fabric dyed with reactive lemon yellow-CN for combined dyed and fragrance finished fabric as well as conventional two stage dyed and fragrance finished fabric have been assessed and shown in Table-1. Relevant data in Table 1 indicate that K/S value, color fastness rating, Light fastness to understand the feasibility of combined dyeing and finishing in compare to conventional two stages dyeing and finishing using Reactive-Lemon yellow CN, Citric acid, synthetic fragrance chemicals-Jasmine and auxiliaries such as beta cyclodextrin/Dextrin.
Table 1. Analysis of dyeing properties of conventional two stage dyeing and finishing as well as simultaneous dyeing and finishing on cotton fabric.
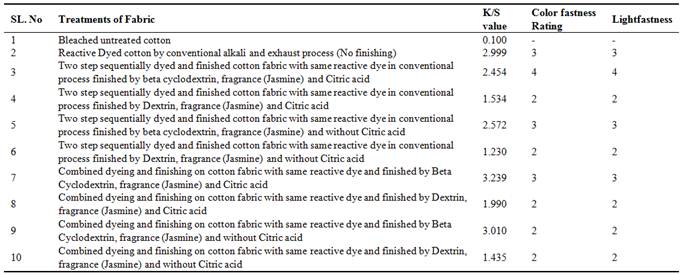
The color strength(K/S) value (shown in Table-1) of combined dyed and finished cotton fabric have been found better than two stage conventional process for all three dosage of beta-cyclodextrinas indicated by k/s value while beta cyclodextrin showed higher k/s value for both combined and two stage process than that for dextrin. Combined dyeing and finishing on cotton fabric obtained deeper color yield for reactive dyeing with monochlorotriazine group of reactive dye using beta cyclodextrin, citric acid & sodium hypophosphite and fragrance finish applied simultaneously. It is known that the said reactive dye forms covalent bond with cellulose and beta cyclodextrin. Due to the covalent bonding between dye, fibre and beta cyclodextrin, it also show at par / marginally level of color fastness to wash and light (shown in table-1).
In conventional method of dyeing and finishing, the major drawback of reactive dyes is hydrolysis in which dye instead of reacting with fibre reacts with hydroxide ion of base, as a result high amount of wastage of dyestuff occurs in conventional dyeing. But in combined dyeing and finishing with padding method hydrolysis rate of dyestuff is being minimized and wastage of dyestuff therefore negligible [29], resulting higher colour strength. So this simultaneous dyeing and finishing work is more beneficial to minimizing the wastage of dyestuff, rendering higher colour strength and overall at par level of fastness to wash and light.
K/s value was improved 31% for beta cyclodextrin in combined dyeing and finishing method. As the price of dyestuff is the highest among all chemical of dyeing and finishing process so it is a worth achievement.
3.2. Study on Physical Properties of Dyed and Finished Cotton Fabric
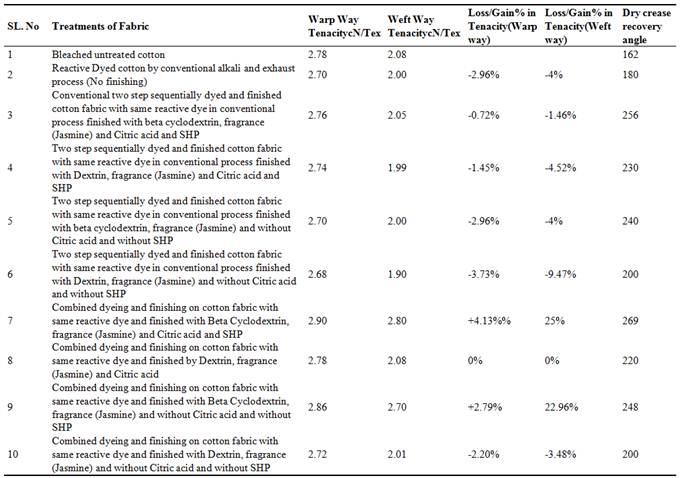
Bleached cotton fabric has been dyed and fragrance finished in simultaneous single stage operation as well as in conventional two stage process. Table-2 shows the different physical properties in combined dyeing and finishing on cotton fabric as well as conventional two stage dyeing and finishing on cotton fabric studied various types of properties such as Fabric strength, Dry crease recovery angle to observe the feasibility of combined dyeing and finishing comparing with double stage dyeing and finishing using Reactive-Lemon yellow CN-1%, Citric acid-10%, synthetic fragrance chemicals (Jasmine): 1% and auxiliaries such as beta cyclodextrin/Dextrin.
Combined dyeing and finishing with beta cyclodextrin was successfully improved the dry crease recovery angle (shown in table-2) of cotton fabric which is a major benefit of using beta cyclodextrin. Beta cyclodextrin may be improving (shown in table-2) the molecular chain in the amorphous region & improved the weak hydrogen bond. Similarly, Dextrin also improved the dry crease recovery property (shown in table-2) may be its similar function of beta cyclodextrin as dextrin is a mother chemical of beta cyclodextrin.
Table 2. Analysis of physical properties of conventional two stage dyeing and finishing as well as simultaneous dyeing and finishing on cotton fabric (for reactive dyeing and fragrance finishing) using beta-cyclodextrin and citric acid with SHP as catalyst.
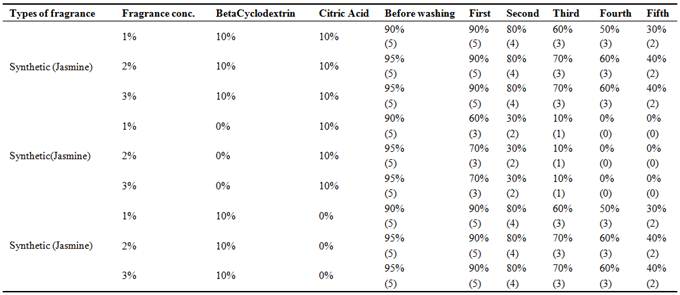
3.3. Study on Fragrance Intensity of Dyed and Finished Cotton Fabric
Fragrance Intensity testing of the cotton fabric was tested by Human sensorial evaluation (Shown in Table-3) Gas chromatography testing (Capillary method) For analysis fragrance intensity in combined dyeing and finishing method were carried out to study the fragrance intensity in double stage dyeing and finishing. It was also studied to know fragrance intensity in two conditions. Such as: after number of days without washing and after five time washing. Finally it was concluded that fragrance on combined dyeing and finishing has been found higher intensity than double stage dyeing and finishing, so details study on fragrance intensity has been proceeded only for combined dyeing and finishing for showing specific concept of retention% of fragrance chemical in fragrance grading scale 0-100% (Shown in Table-3) was experimented by human sensorial evaluation (by systematic evaluation of the fragrance and opinion of 10 different persons) after number of days without washing. Treated by Reactive-Lemon yellow CN-1%, Beta Cyclodextrin-10%, Citric acid-10% and synthetic fragrance chemicals (Jasmine-1%) were found good intensity of fragrance which was tested by Gas chromatography (Shown in Graph -1&2) method for further confirmation of fragrance retention. Human sensory evaluation (Shown in Table-3) was also confirmed fragrance retention/microencapsulation on cotton fabric of synthetic Jasmine was retained up to 100 days without washing where beta cyclodextrin was used 10% but fragrance finishing without beta cyclodextrinretained up to 30 days.
Table 3. Fragrance retention grading scale (0-100%)by human sensorial evaluation by opinion pole after number of days without washing upto 100 days for sample no: 7 with 20-40 Beta cyclodextrin, citric acid and SHP.
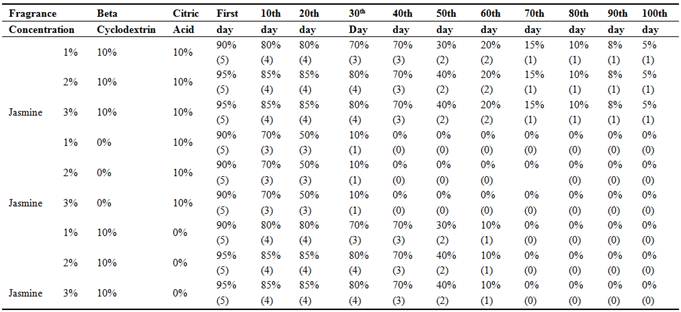
Table 4. Fragrance retention grading scale (0-100%)by human sensorial evaluation by opinion pole after number of days without washing upto five time washing by using neutral soap-2gm/l, time-3min, RPM-40, Temp-40°C for sample no: 7 with or without Beta cyclodextrin and with or without citric acid.
3.4. Study on Gas Chromatography for Fragrance Intensity Testing Without Washing
In combined dyed and finished cotton fabric which was treated by Reactive-Lemon yellow CN-1%, beta-cyclodextrin-10%, Citric acid-10% and synthetic fragrance chemicals (Jasmine-1%) and tested by gas chromatography method (Shown in Fig.-2). For testing GC, firstly standard Jasmine was tested shown in Fig.-1 and secondly fragrance treated fabric was tested by extraction method shown in Fig.-2. The intensity of jasmine fragrance retained after 100 days without washing in cotton fabric is 39.5%. This percentage was found by the calculation method of area%. Since combined dyed and finished cotton fabric was extracted by acetone and standard fragrance chemical also mixed with acetone, So almost similar rectangular curve showed for both bleached and treated cotton fabric. Fragrance retention time was gradually decreased in case of treated sample. Technical language of another Y-axis curve is called T-peak and another small vibration due to the use of synthetic fragrance. An extended area was showed in the Fig.-2 of standard Jasmine due to mixed of unknown substance in synthetic fragrance where may be used varieties quality of natural and artificial fragrance to produce synthetic fragrance of Jasmine.
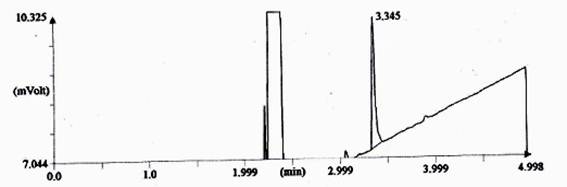
Fig. 1. Chromatograph of fragrance intensity of synthetic Jasmine as standard.
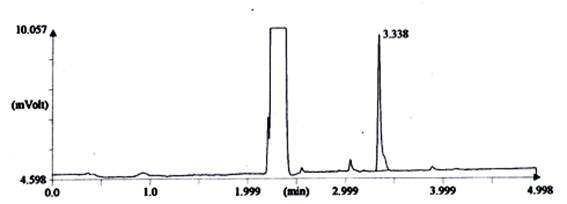
Fig. 2. Chromatograph of fragrance intensity (synthetic Jasmine) on combined dyed and fragrance finished cotton fabric after 100 days without washing.
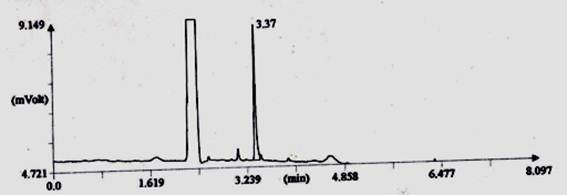
Fig. 3. Chromatograph of fragrance intensity as standard synthetic Jasmine.
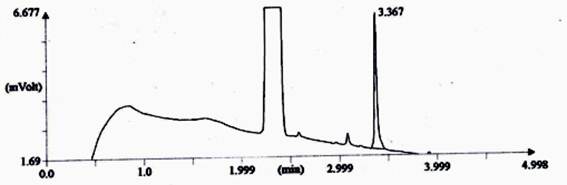
Fig. 4. Chromatograph of fragrance intensity (synthetic Jasmine) on combined dyed and fragrance finished fabric after five time washing.
3.5. Study on Gas Chromatography After Five Time Washing
In combined dyed and finished cotton fabric which was treated by Reactive-Lemon yellow CN-1%, Beta-cyclodextrin-10%, Citric acid-10% and synthetic fragrance chemicals (Jasmine-1%) and tested by gas chromatography method (Shown in Fig. 4). For testing GC, firstly standard Jasmine was tested shown in Graph-3 and secondly fragrance treated fabric was tested by extraction method shown in Fig. 4. The intensity of jasmine fragrance retained after five time washing in cotton fabric is 15.88%. This percentage was found by the calculation method of area%. Since combined dyed and finished cotton fabric was extracted by acetone and standard fragrance chemical also mixed with acetone. So almost similar rectangular curve showed for both bleached and treated cotton fabric. Fragrance retention time was gradually decreased in case of treated sample. Technical language of another Y-axis curve is called T-peak and another small vibration due to the use of synthetic fragrance.
Thus from Gas Chromatography analysis it may be concluded that combined dyeing and finishing can retain good amount of fragrance 39.5% after 100 days without washing due to the presence of beta cyclodextrin as microencapsulation as well as crosslinking of citric acid and similarly retaining15.88% fragrance was observed after five time washing due to the presence of beta cyclodextrin as microencapsulation as well as crosslinking of citric acid.
3.6. FTIR Study of Combined Dyed and Fragrance Finished Cotton Fabric
Fourier transform infrared (FTIR) spectra analysis for (a)Bleached cotton fabric (shown in Fig.-5) as well as (b) combined dyed and fragrance finished of cotton fabric treated with Reactive-Lemon yellow CN-1%, beta-cyclodextrin-10%, Citric acid-10%, 2% SHP and synthetic fragrance chemicals (Jasmine-1%)3305.91 cm-1 peak (Shown in Fig.-6) indicates (-OH) group where clearly showed more extended area of (-OH) group in combined dyed and finished fabric than bleached cotton fabric (shown in Fig.-5). From where it may be assumed that (-OH) group of cellulose is bonding with the (-OH) group of beta cyclodextrin. From 1600 cm-1 to 1000 cm-1 no new peak has been formed but a new peak fully removed 983.62 peaks from the graph-6 of bleached cotton fabric which may be due to some reaction among monochlorotriazine group of reactive dye, synthetic fragrance chemical, cellulosic fibre and citric acid. (Shown in Fig.-6) for combined dyeing and finishing fabric a new absorption curveappeared1728 cm-1 (C=O) and the C-H stretching vibration 2902.38 cm-1 was improved slightly due to the improvement of macromolecular chain. The new strong absorption peak 1728 cm-1, 902.03 cm-1, 819 appeared due to C=O stretching vibration, which was accord with the theory of cotton fabric treated by beta cyclodextrin and citric acid. Ester bond (-COO-) was generated between hydroxyl group of beta cyclodextrin and cotton fabric reacted with citric acid which was used as crosslinking agent. Fig.-2 showed that beta cyclodextrin was fixed on cotton fabric with the aid of citric acid which crosslinking among the carboxyl group of acids, the hydroxyl group of cellulose and beta cyclodextrin occurred due to the esterification reaction where the new strong absorption peak 1728 cm-1. So it was speculated cotton fabric was cross linked successfully. Finally it may be assumed that beta cyclodextrin and fragrance chemical are not making any negative reaction with monochlotriazine group of reactive dyestuff. Although, jasmine the fragrance oil was microencapsulated primarily before making any reaction with fibre and the strong absorption peak (Shown in Fig.-6) confirmed that beta cyclodextrin was bonding with monochlorotriazine group.
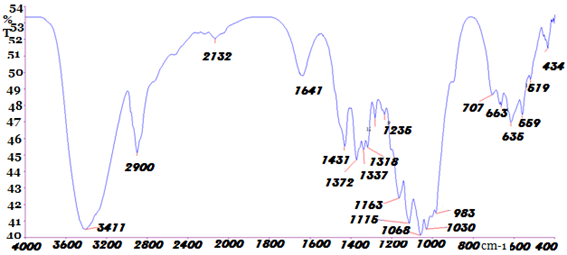
Fig. 5. FTIR of bleached cotton fabric.
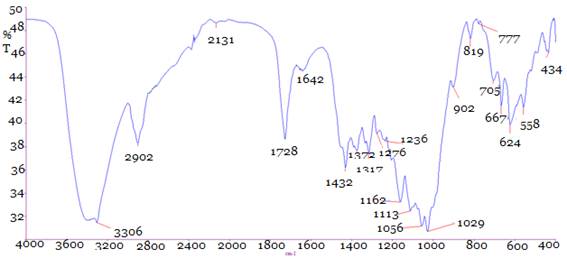
Fig. 6. FTIR of combined dyed and finished fabric.
4. Conclusion
From the present study it is revealed that combination of dyed and microencapsulated aroma finished fabric shows adequate wrinkle resistance, high tensile strength retention property and higher colour value of cotton fabric and also rendered high retention of aroma property as indicated by gas chromatography test and human sensorial evaluation as compared to untreated fabric as well as compared to sequential two step process of dyeing and finishing. The optimum performances were obtained with Synthetic Fragrance (Jasmine): 1%, Reactive dye-lemon yellow-CN: 1%, Beta Cyclodextrin: 10%, Citric Acid: 10%, Sodium Hypophosphite: 6%, Solvent composed of PEG & Water in ratio 1:3:72%.
Irrespective of the technique followed, the surface colour strength of simultaneous dyed and aroma finished cotton fabric is found to increase with an increase the percentage of application of both beta-cyclodextrin from and beyond which it almost tends to level off. The fastness parameters are also moderately satisfactory.
Single step simultaneous dyeing (with reactive dye) and aroma finishing of cotton fabric by pad-dry-cure technique thus offers savings in energy, process time, giving satisfactory colour strength and acceptable other physical parameters as compared to the conventional two-step sequential dyeing and aroma finishing process. Hence this process may be implemented industry for savings of time/ energy/ labour and process cost etc.
References
- Bartolomeo, J. Aromarama. Women’s Sports & Fitness. 1999, 3(11-12):84.
- Mazzaro, D. 2000. The home fragrance market. Chemical Market Reporter, Au-gust 4:14.
- Usha, Rashmi, Bhaskara-Amrit, Pramod B. Agrawal and Marijin M. C. G. Warmoeskerken, Application of B-cyclodextrins in textiles, AUTEX Research journal, Vol.11, (4) December 2011.
- Dr. Charles Tomasino, Dept. of textile engineering, North Carolina state university, Chemistry and technology of fabric preparation and finishing, Pages: 17, 50, 102-106, 109-114, 130,154-162, 164-170, 219.
- Matsushi-Shikiso Chemical Co. Ltd. Fixing Odourous to Textiles. High-Per-formance-Textiles. 1994(8):7-8.
- DevinderKaur and NeelamGrewal, Effect of ecofriendly microencapsulated textile products in joint pain and aesthematic respondents, Journal of polymer and textile engineering,Vol.1, Issue 2, PP:41-45, January 2014
- J.-P. Moldenhauer, H. Reuscher, Textile finishing with MCT-Beta cyclodextrin, in Proc. 9th Int. Symp. Cyclodextrins (1999), (Eds. J. Torres Labandeira, J. L. Vila-jato), Kluwer Academic publishers, Dordrecht, 1998, pp. 161-165.
- David Rowe, WILEY publications, Chemistry and technology of flavours and fragrances.
- M. Sundrarajan and Rukmani, Durable antimicrobial finishing on organic cotton by inclusion of thymol into cyclodextrin derivative, E-journal of chemistry, 2012, 9, 1511-1517, http://www.ejchem.net.
- Aurelia Grigoriu and Octavian Popescu, Application of cyclodextrines in textiles – a review, Buletinulinstitutuluipolitehnic din iasi, Publicatde, UniversitateaTehnica" Gheorghe asachi" din Iasi, Tomul LVII, (LXI), Fasc.2,2011.
- Bushmann, H-j. Removal of Residual surfactant deposits from textile materials with the aid of cyclodextrins. MelliandTextilberchite.
- J. Koch, Stabilisation and controlled release of perfume in detergents, in proc. 1st Int. Symp. Cyclodextrins (Ed.:J. Szejtli) Reidel, Dordrecht,1982, P.487
- B. Martel, M. Wetrowski, D. Ruffin, M. morcellet, Polycarboxylic acids as crosslinking agents for grafting cyclodextrins onto cotton and wool fabrics: study of the process parameters, J. Appl. Polym. Sci 2002, 83(7), 1449-1456.
- H.-J. Buschmann, D. Knittel, E. Schollmeyer: Use of cy-clodextrins for improving waste air washers, MelliandTextil-ber.2001, 82(5), E94-E96, 368-370.
- H.-J. Buschmann, D. Knittel, E. Schollmeyer, New textile applications of cyclodextrins, J. Inclusion Phenom. Macrocyclic Chem. 2001, 40(3), 169-172.
- Katia Johansen, Perfumed textiles, Textile society of America symposium proceedings, Paper 104 September 4-7, 2008,http://disitalcommons.unl.edu//tsaconf/104.
- Stephen Warrenburg, Effects of fragrance on emotions: Moods and physiology, International flavors and fragrances Inc, 1515 Rt. 36, UnionBeach, NJ07735, USA, email: Stephen. Warrenburg @ iff.com.
- Aromatextiles, http://textilelearner.blogspot.com/2013/03/aroma-textile-application of html #ixzz3lkzlxk00.
- Shirley Institute, New finishes using microencapsulation, Textile month, 1988(5): 49-49, Mei W-P. Application of Microencapsulation, Technology in textile coloration and finishing, Journal-china-Textile-Institute.1995.5 (3):188-191.
- Leena Mishra, Dept. Of Fibres & Textile Processing Technology, University Of Mumbai, Institute Of Chemical Technology, microencapsulation: An overview & its application in textile wet processing.
- S.Y. cheng, C. W. M. Yuen, C. W. Kan and K. K. L. Cheuk, Development of cosmetic textiles using microencapsulation technology, RJTAVol.12 (4) 2008.
- Ramniksingh, NitinBharti, Jyotsana, Madan, S. N. hiremath, Characterization of cyclodextrin inclusion complexes-a Review, Khalsa college of pharmacy, Amritstar-143302, Punjab, india, Sri Sri college of pharmacy, Badhani, Pathankot-145001(Punjab, Pravara rural educationsociety’s college of pharmacy, Chincholi, Nashik-422101.
- C. X. Wang, Sh. L. Chen, Aromachology and its application in the textile field, email: Wangchaoxia@sohu.com, College of textile and garments, Southern Yangtze University, College of chemistry and Chemical engineering, Donghua University, Shanghai 200051, P.R. China .
- Buschmann, H-J, MelliandTextilberchite, Cyclodextrins and dextrins as new auxiliaries in dyeing, 1991, 72 (12): 1012-1014.
- Jozsef Szejtli, Cyclodextrin in the textile industry, CYCLOLAB, Research and development laboratory ltd., Budapest, Hungary, Starch/Starke 55, (2003) 191-196.
- Anjali Karolia, Snehal Mendapara, Imparting antimicrobial and fragrance finish on cotton using chitosan with silicon softner, Indian journal of fibre and textile science,Volume-32, March-2007, PP-99-104.
- D. P. Chattopadhyay, Shweta, J. Doctor, Synthesis of reactive fragranced entities and their application to cotton textiles, Textiles and light industrial science and technology, Volume-2, Issue-2, April-2013.
- Improvement of ink jet printing performance using beta cyclodextrin on cotton fabric, Textile research journal, Manuscript ID TRJ-15-0179.
- M Kamaluddin, ME Molla, S M BRahman, M A K Azad, H M ZakirHossain & M MU Jubayer, Simultaneous dyeing and finishing, J BioSci, 7(1) (2007)68-70.
- D. P. Chattopadhyay, Shweta J. Doctor, Synthesis of Fragranced Dyes and Their Application to Cotton Textiles, Journal of Textile and Apparel Technology and Management, Volume 8, Issue 2, September 2013.
- Ahmed G Hassabu, Research Journal of Textile Apparels, January 2013.
- Cristian Dima, Mihaela Cotarlet, Balaes Tiberius, Gabriella Bahrim, Petru Alexe, Stefan Dima, Encapsulation of coriander essential oil in beta cyclodextrin: Antioxidant and Antimicrobial Properties Evaluation, Romanian biotechnological letters, Vol 19, No. 2, 2014.

 (A. K. Samanta)
(A. K. Samanta)  (K. Bhattacharya)
(K. Bhattacharya)  (A. Hossain)
(A. Hossain) 











